Two studies look at trends in the cost and length of time spent developing new pharmaceuticals
Explore This Issue
July 2006Since 1994, US spending on prescription drugs grew three times faster than healthcare spending as a whole. That affects payers, plans, patients, and physicians. Pharmaceutical companies claim that the cost of developing a new molecular entity (NME), often exceeding $1 billion, justifies high prescription drug prices. Two recent articles scrutinize that claim, examining the true costs of pharmaceutical innovation.
Development Costs: Is There One Answer?
Quantifying the cost of developing a new drug is a crucial question in the drug development debate. A recent analysis by Christopher P. Adams and Van V. Brantner entitled “Estimating the Cost of New Drug Development: Is It Really $802 Million?” notes that there is great variation in actual development costs for new drugs (Health Affairs 2006; 25 (2):429–436).
According to the authors, drug development costs averaged $868 million per NME, with a range of $500 million to over $2 billion (in year 2000 dollars), depending on therapeutic classes and the developing company. The authors compared their results with an earlier study by Joseph A. DiMasi and colleagues (Jrnl of Health Economics 2003: 151–185) that put the average drug development cost at $802 million, attributing the discrepancy of their findings to different data sets. They also noted that clinical costs of research and development (R&D) were fairly consistent with DiMasi’s data; $487 million for the new study versus $467 million for DiMasi.
When looking at the largest US pharmaceutical firms, Adams and Brantner found that firms developing the most new drugs outspent smaller rivals by $124 million for an NME. There were also significant differences in drug R&D by firm. One company developed 92 drugs at an average cost of $2.1 billion, as opposed to 34 drugs at an average cost of $521 million at a similar size firm.
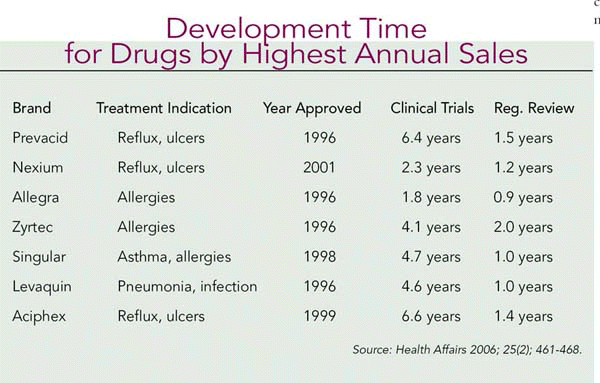
Drugs with annual projected sales of $1 billion or more took one year less to develop than drugs with lower profit potential.

Physician’s Perspective
Michael Goldrich, MD, of University Otolaryngology Associates in New Brunswick, NJ, who is a general pediatric and adult otolaryngologist with a subspecialty in laryngology, said he doesn’t object to pharmaceutical companies’ R&D spending because companies are investing in the future of clinical care.
What does bother him is the pharmaceutical industry’s huge investment in direct to consumer (DTC) advertising; “They are using the patient as a proxy for the physician since government oversight of drug companies’ contacts with physicians has intensified,” said Dr. Goldrich. “I’m very cognizant of that change, and vigilant that my decisions about what to prescribe are never influenced by what the patient has been bombarded with through drug company marketing,” he added. That means more of his time spent dispelling the marketing messages that patients receive from drug advertising.
The sense that heavy DTC advertising for new drugs inflates overall spending is reflected in a December 2003 study by Bain & Co. that puts an NME’s cost at $1.7 billion, 55% higher than the average from 1995 to 2000. It cited skyrocketing marketing budgets for new drugs as largely responsible for higher R&D and prescription drug costs. The Bain study included 12 months of sales and marketing costs incurred in launching a new drug.
Dr. Goldrich also keeps abreast of a drug’s efficacy and performance, particularly when its patent protection expires and competing firms produce generic equivalents. “For a while Claritin was extremely popular in its class, but when it went OTC [over the counter], the manufacturer stopped promoting it heavily. Now there are new and better drugs,” he said.
This example highlights the complex relationship between drug R&D, patent protection, and the cost of patients’ medications. Schering-Plough got its money’s worth from Claritin, according to Marcia Angell, MD, in her 2004 book, The Truth about Drug Companies (Random House, 2004): before the blockbuster drug’s patent expired, the firm raised Claritin’s price 13 times in five years, a 50% cumulative increase.
When a drug such as Claritin loses patent exclusivity and becomes available without a prescription, physicians and patients face a dilemma. Use the same formulation, now OTC, and face higher out of pocket costs because the drug is no longer a covered prescription drug? Switch to a generic equivalent with a low co-pay? Choose a newer drug that may be more effective and more expensive?
Drug R&D and Drug Prices
Another pertinent question in the drug R&D cost debate is development time. Are new drugs getting to market more quickly and cost-effectively than in the past?
The pharmaceutical industry argues that the long R&D process drives high drug prices. However, Salomeh Keyhani, MD, and colleagues found that clinical trial periods have not increased between 1992 and 2002, and that regulatory review times actually decreased during that period in their review of development times for 168 NMEs (Are Development Times for Pharmaceuticals Increasing or Decreasing? Health Affairs 2006; 25(2); 461–468).
When comparing drug development times between 1985 and 1995, the average time was just over 10 years. For 1992 through 2002 the authors reported the median times for clinical trials and regulatory review were 5.1 years and 1.2 years, respectively. They conclude that the relative value of drugs in their therapeutic classes and market dynamics have more impact on cost than drug development times. Drugs with annual projected sales of $1 billion or more took one year less to develop than drugs with lower profit potential. (See Development Time for Drugs by Highest Annual Sales, above, for the clinical trial and regulatory times for some of the last decade’s blockbuster drugs.)
According to the authors, drug development costs averaged $868 million per NME, with a range of $500 million to over $2 billion, depending on therapeutic classes and the developing company.
Better Processes Can Encourage Innovations
Dr. Keyhani and colleagues also propose that improvements in the regulatory process, particularly FDA “fast tracking” of drug approvals, allow for speedier trials and lower clinical trial costs. The same legislation that allowed for fast tracking also permits faster development of orphan drugs.
The streamlined regulatory process has allowed Zila, Inc., a small pharmaceutical company based in Phoenix, to move swiftly with OraTest, an oral cancer-specific rinse being evaluated for its efficacy in early detection of oral cancers in high risk patients.
Zila received a special protocol arm from the FDA for Phase III clinical trials, which accelerates the trials and allowed Zila to cut its R&D costs by enrolling fewer than 4,000 high-risk patients in 13 sites including Johns Hopkins University, M.D. Anderson Cancer Center, and New York University’s College of Dentistry. Zila’s CEO Douglas Burkett, PhD, hopes that OraTest will improve oral cancer detection. “Currently, almost 70% of oral cancers are detected late in the disease, leading to low five-year survival rates. If the OraTest clinical trial succeeds, we hope to improve the survivability of this cancer,” said Dr. Burkett.
Still, the most recent research indicates that drug development times may not be a factor in rising drug prices, and that the costs of new drug development vary widely, from $500 million all the way up to $2 billion. While we may not like high prescription drug costs, Dr. Goldrich reminds us “high drug R&D costs are the price of progress.”
©2006 The Triological Society
Leave a Reply