Explore This Issue
July 2013What’s the best way to evaluate dysphonia? That question has been the subject of much discussion and research in otolaryngology since the American Academy of Otolaryngology – Head and Neck Surgery released the first and only clinical practice guideline for hoarseness (dysphonia) in 2009 (Otolaryngol Head Neck Surg. 2009;141:S1-S31).
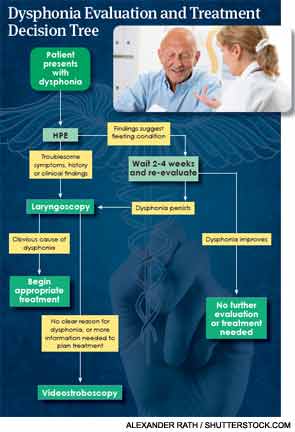
While the guideline states that a “clinician may perform laryngoscopy at any time in a patient with hoarseness,” it also includes a strong recommendation that states that “the clinician should visualize the patient’s larynx, or refer the patient to a clinician who can visualize the larynx, when hoarseness fails to resolve by a maximum of three months after onset, or irrespective of duration if serious underlying cause is suspected.”
However, some otolaryngologists argue that waiting three months, even if serious disease is not expected, may delay appropriate treatment, negatively affect patients’ quality of life and increase health care costs. “If a patient walks into an ophthalmologist’s office saying they can’t see, you don’t just give them glasses and say, ‘OK, see how this goes for three months.’ You do an exam to find out why the patient can’t see and then you give them the treatment,” said Milan Amin, MD, associate professor of otolaryngology at New York University School of Medicine and director of the NYU Voice Center.
To effectively diagnose and treat voice disorders, otolaryngologists have to get down to the root cause of the difficulty. “Hoarseness is a symptom,” said Clark Rosen, MD, associate professor of otolaryngology at the University of Pittsburgh and director of the University of Pittsburgh Voice Center. “Our job is to create a differential diagnosis and work through that differential diagnosis.”
Initial Evaluation of Voice Disorders
Some causes of voice disorders are obvious and self-limiting. “If I go to a Knicks game and yell, I’m going to get hoarse, and that’s a relatively fleeting condition. If I have an upper respiratory infection and I’m hoarse, there’s reason to believe that’s a fleeting condition,” said Ryan Branski, PhD, assistant professor of otolaryngology at New York University and associate director of the NYU Voice Center.
But beyond such obvious, common causes of dysphonia, relying on a basic history and physical to diagnose and treat voice disorders—even for an initial three-month period—may be dangerous. A 2013 study compared the diagnostic accuracy of history, laryngoscopy and stroboscopy and found that a history and physical exam (HPE) led to a correct diagnosis just 5 percent of the time. The accuracy of diagnosis increased to nearly 70 percent after visualizing the larynx with flexible laryngoscopy and videostroboscopy. Laryngoscopy and stroboscopy did not miss any cancer diagnoses, while clinicians missed five cases of cancer based on HPEs (Laryngoscope. 2013:123:215-219).
The article did not assess the accuracy of diagnosis when mirror examination of the larynx was included as part of the initial exam, because primary care doctors—a major target audience of the 2009 clinical practice guideline—are not able to perform mirror laryngoscopy. “The article studied history and simple physical exam alone, and did not include mirror exam or even sound samples of the voice, due to technical reasons. This is also pretty much what primary care doctors do when they see a patient with hoarseness,” Dr. Amin said. “Mirror exam (and to some degree, listening to the voice) may increase diagnostic accuracy. The point of the article is that history is a poor guide to diagnosis, and the hoarseness clinical practice guideline uses history to stratify patients according to risk levels. But if history is a poor guide, then recommendations based on history alone are not helpful. Somebody should get eyes on that larynx within a relatively short period of time. Nobody would ever treat a skin rash without looking at it, or treat glaucoma without measuring the pressure. How do you treat a laryngeal condition if you have no idea what you’re treating?”
Most practicing otolaryngologists, including some who helped draft the guidelines, believe that waiting three months before visializing the larynx is unreasonably long. “If a voice problem is lasting more than two or three weeks, then that’s not acute laryngitis, and you don’t know what it is unless you look at the larynx,” said Seth Cohen, MD, MPH, associate professor of surgery in the division of otolaryngology-head and neck surgery at Duke University and an author of the 2009 guidelines.
Laryngoscopy
Using a flexible laryngoscope is an easy, relatively low-cost way to visualize the larynx, and the risks to the patient are minimal. A 2012 study of 250 patients found that it is also well tolerated by patients (Ann Otol Rhinol Laryngol. 2012:121:708-713). Given the advantages of laryngoscopy, it “should be a bare minimum for somebody with a voice complaint lasting longer than two or three weeks,” Dr. Rosen said.
Waiting longer increases the risk that significant pathology might be missed, said Mark Courey, MD, director of laryngology and the medical director of the UCSF Voice and Swallowing Center. “Unpublished data suggests that when a patient’s referral for laryngoscopy is delayed and they have remained dysphonic for three to six months, they are significantly more likely to have a diagnosis of cancer made on their initial visit to an otolaryngologist,” he added. “I see probably one patient every other month whose referral was delayed a year, and now I’m taking out their larynx as opposed to treating an early cancer and preserving their larynx.”
Delaying laryngoscopic evaluation may also contribute unnecessarily to patients’ decreased quality of life. “Voice problems are not cosmetic,” Dr. Cohen said. “Even if cancer isn’t the cause, people with dysphonia suffer work disability, can’t communicate and become socially isolated. We wouldn’t expect somebody with back pain, for example, to wait three months before an evaluation.”
Performing laryngoscopy earlier allows for appropriate treatment and may decrease medication-related morbidity and health care costs. “Compare what a laryngoscopy costs, which through Medicare is about $200, versus a single month prescription of Nexium, which is often prescribed for supposed acid reflux and costs a few hundred dollars. Some patients are put on three or four months of Nexium or a proton pump inhibitor, so that’s a significant difference,” Dr. Amin said.
While most otolaryngologists understand that laryngoscopy is always preferable to computed tomography (CT) or magnetic resonance imaging (MRI) for initial visualization of the larynx, some health care practitioners still order CT scans or MRIs before referring patients for laryngoscopy. Otolaryngologists can help primary care doctors understand that “CT scans are terrible for looking at laryngeal pathology,” Dr. Amin said. “You get a much more sensitive view with a laryngoscope.”
Videostroboscopy
If laryngoscopy reveals a blatantly obvious cause for a patient’s voice problems, “you can stop there,” Dr. Rosen said. But if there is no obvious cause for the problem, videostroboscopy may be warranted.
However, videostroboscopy is not routinely performed as the next step in the evaluation of voice disorders. A 2012 study of the practice patterns of 982 otolaryngologists revealed that 57.9 percent obtained stroboscopy for adult dysphonic patients with no vocal fold lesions and normal vocal fold mobility, 58.6 percent prescribed a proton pump inhibitor, 63 percent initiated a referral to speech pathology, 11.7 percent referred to a laryngologist and 9.2 percent prescribed an oral steroid (respondents were allowed to select more than one answer) (Otolaryngol Head Neck Surg. 2012;147:289-294).
Yet the best available data shows that videostroboscopy often changes diagnosis and treatment. “The research shows that roughly 10 to 47 percent of diagnoses change or are modified after you go from laryngoscopy to stroboscopy in tertiary care voice clinics,” Dr. Cohen said.
“Is a stroboscopy absolutely necessary? Under certain circumstances, I think it is,” Dr. Courey said. You wouldn’t perform a middle ear operation to replace the ossicles without doing an audiogram, right? You may know that the patient has a conductive hearing loss test because of a tuning fork test, but what otologist is going to operate without specifically defining what the conductive hearing loss is? For laryngologists, the laryngoscopy is like our tuning fork. Stroboscopy is our audiogram.”
Of course, videostroboscopy is not appropriate or necessary for all patients with voice complaints. “If a patient presents with a very large polyp that’s creating dysphonia and just wants to know that it’s not cancer, you document that in the chart and ask the patient, ‘Do you want to look at this further?’ If the patient specifically says no, then delaying the stroboscopy is appropriate. However, I think you should document that discussion,” Dr. Courey said.
 “You wouldn’t perform a middle ear operation to replace the ossicles without doing an audiogram, right? You may know that the patient has a conductive hearing loss test because of a tuning fork test, but what otologist is going to operate without specifically defining what the conductive hearing loss is? For laryngologists, the laryngoscopy is like our tuning fork. Stroboscopy is our audiogram.”
“You wouldn’t perform a middle ear operation to replace the ossicles without doing an audiogram, right? You may know that the patient has a conductive hearing loss test because of a tuning fork test, but what otologist is going to operate without specifically defining what the conductive hearing loss is? For laryngologists, the laryngoscopy is like our tuning fork. Stroboscopy is our audiogram.”
—Mark Courey, MD
Working with Speech-Language Pathologists
Videostroboscopy is performed either by otolaryngologists or speech-language pathologists (SLPs). “Speech pathologists look at things from a physiologic perspective,” said Dr. Branski. “We try to determine if the patient is stimulable for voice therapy. Physicians tend to look at it from a straightforward anatomical perspective.” Because both health care specialties have a slightly different focus, it’s in patients’ best interests for otolaryngologists and SLPs to collaborate—and absolutely necessary for both to view the video of the exam (not just the still photos), no matter who performed the videostroboscopy.
“No otolaryngologist would operate on sinus disease without having the CT scan in the OR and having looked at all of the images available on that CT. In the same way, you should not operate on the larynx unless you have looked at and interpreted the entire videostroboscopy,” Dr. Courey said. “There are no official guidelines on this, but why would you apply a different standard?”
Close collaboration between otolaryngologists and SLPs can help patients avoid surgery or obtain optimal surgical results. “I may know that I could use surgical methods to resolve a patient’s problem, but sometimes, voice therapy may be all the patient needs or wants,” Dr. Courey said. “And if a patient is straining in a way that contributes to their voice problem, having the patient learn to stop straining is going to make my surgery much easier and more effective.”
Viewing the videostroboscopy helps SLPs plan voice therapy effectively. “Voice therapy today is physiologically based,” said Edie Hapner, PhD, CCC-SLP, assistant professor and director of speech language pathology at Emory University in Atlanta. “Up until the 1990s, we did symptomatic voice therapy. If you were too loud, we made your voice quieter. If you were too quiet, we made you louder, but we had no idea what was going on in the vocal folds. Now, we have the tools to look at them, so in order to design the most effective voice therapy, I need to understand what’s happening at the level of the vocal folds.”
Videostroboscopy can also be used to objectively document the success of vocal therapy, especially when the goal of therapy is to reduce a lesion’s impact or to reduce the size of the glottal gap.
“Laryngoscopy and stroboscopy are not new technologies, so many physicians are drawn to more complicated examinations like a CT scan, MRI, esophagoscopy, pH probe and testing for reflux before looking at the larynx,” Dr. Rosen said. “But we can use some simple, very valuable, effective techniques and find the actual cause of hoarseness by using laryngoscopy plus or minus a stroboscopy evaluation.”
Leave a Reply