Maureen Hannley, PhD, is currently Chief of the Research Division of the Department of Otolaryngology and Communication Sciences at Medical College of Wisconsin and Research Consultant for the Triological Society. She formerly served as the Chief Research Officer of the American Academy of Otolaryngology- Head and Neck Surgery Foundation and has held positions at the National Institutes of Health, Stanford University Medical School, and Arizona State University.
Explore This Issue
January 2008How are your patients doing? Do you know the impact of their disorders-and the management approach you selected for them-on their health-related quality of life? An audiogram may tell you a patient’s type, degree, and configuration of hearing impairment, but it doesn’t tell you the impact of that impairment on her emotional status because she can no longer play cards with her friends, participate in conversations in a noisy restaurant, or hear the secrets whispered to her by her granddaughter. Polysomnography will yield an apnea-hypopnea index, but does not reflect the ways in which sleep-disordered breathing can affect how much energy a person has, how he manages in the workplace, or how he functions behind the wheel of a vehicle. Such information must come directly from the patient and it is highly individual.
 Outcomes research is concerned with effectiveness, or the impact of the intervention on the overall health and well-being of the typical patient under real-world circumstances.
Outcomes research is concerned with effectiveness, or the impact of the intervention on the overall health and well-being of the typical patient under real-world circumstances.Every clinician is interested in the outcome of his or her treatment, and many providers and consumers of health care believe that patient outcomes should be evaluated as a part of routine patient care. Patient outcomes, however, has evolved into something of an umbrella term used loosely to encompass medical outcomes, health-related quality of life, patient/customer service satisfaction with care, and cost/operations of care. Increasingly, information about symptoms and performance is being gathered directly from patients using structured questionnaires that have been shown to yield meaningful, quantitative assessments of how patients feel and how they function with their disorders and as a result of treatment-measures that are called patient-reported outcomes (PROs).1 In this article we will focus on disease-specific outcomes measures in otolaryngology-head and neck surgery that can be completed by your patients before and after treatment, enabling you to track these very important outcomes with a minimum of disruption to your normal practice routine. This column will focus on selecting the right outcome instrument for your purpose; the next one will describe how to implement an outcomes assessment program in your practice.
Outcomes research seeks to expand upon studies of clinical efficacy, which generally measure the biological effects of an intervention under ideal circumstances in a rigidly defined and controlled group of patients. Outcomes research is concerned with effectiveness, or the impact of the intervention on the overall health and well-being of the typical patient under real-world circumstances. Although the scope of most efficacy studies is necessarily narrow-constrained by the need to maintain control over experimental variables-the scope of outcomes research is broad. For example, whereas efficacy studies would limit a research question to the impact of an intervention on certain biological markers, outcomes research would consider the impact of that same intervention on overall patient health, including physical, social and vocational functioning, quality of life, pain control, symptom relief, emotional status, and satisfaction with care-the parameters that patients typically care about.
Why Measure Patient Outcomes?
There have never been more reasons to measure patient outcomes. The changing health care marketplace is demanding new levels of accountability from providers, health care systems, and health plans. Purchasers and patients want evidence that health care dollars are used for high-quality, effective care-treatment that will improve a person’s health and daily functioning as a whole human being, not just a simple biological system. Patients judge the effectiveness of a drug or procedure by its impact on their lives; they want to know whether the treatment will relieve their most significant symptoms and in the process alleviate anxiety, depression, and frustration. Clinicians need to have better insight into the impact of various management approaches on patients’ long-term clinical status and quality of life. Building a database of patient experiences across treatments and conditions can help provide this type of information-and can help the specialty, future patients, and the individual clinician.
Types of Outcomes Instruments
Health-related quality-of-life outcome measures encompass physical and social functioning, activities of daily living, bodily pain, health perception, and mental health. These measures may be either generic, disease-specific, or intervention-related. Generic health status measures are broadly applicable across practice settings, health conditions, and medical treatments, but are not particularly sensitive to many common conditions in otolaryngology such as mild to moderate hearing impairment, voice disorders, and rhinosinusitis. Some well-known and widely-used generic health status measures include the Medical Outcome Study Short-Form Health Survey (SF-36),2 the Sickness Impact Profile (SIP),3 and the Health Status Questionnaire (HSQ).4
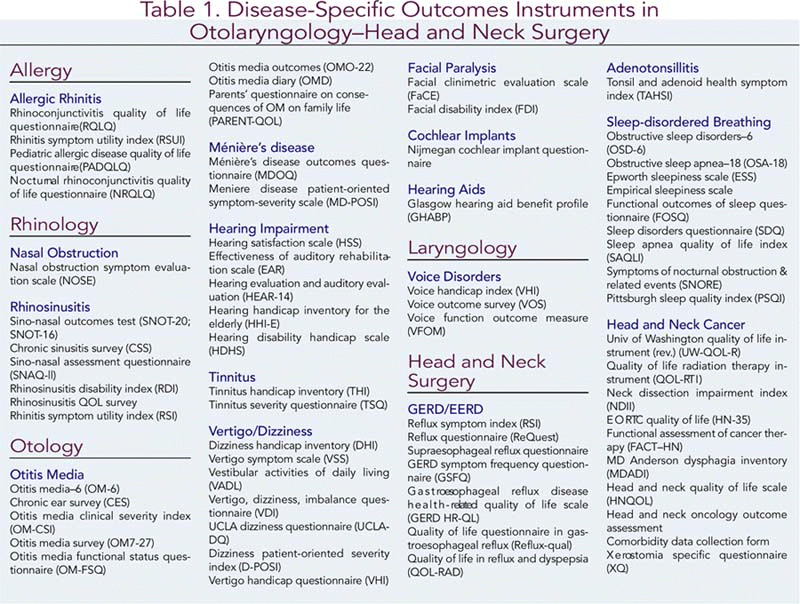
Disease-specific and intervention-related outcome measures have proliferated over the past decade as they are able to capture more detailed data regarding function affected by the underlying condition and make the attribution of clinical change to treatment more straightforward.5 Table 1 lists 68 disease- and intervention-specific outcome measures in otolaryngology-head and neck surgery. Although this list is not comprehensive, many of the common diseases, disorders, and conditions treated by otolaryngologists are represented in the list, offering a choice of instruments for some conditions. All are patient-completed forms (rather than requiring responses through a personal or telephone interview), so that they can be given to your patients for completion prior to the appointment or while in the waiting area of your office. While having the obvious benefit of saving staff time, questionnaires are also less expensive, more uniform, increase patient privacy, and may also increase valid responses when compared to an interview, where patients may feel pressure to give an acceptable response.
How to Choose an Outcomes Instrument
The first criterion for choosing an outcomes instrument for assessment of patients in your practice is what the objective of your study is. What do you want to know-symptom severity and their impact on the patient’s life? The psychological impact of the disorder on the patient and his or her family? The effect of the disorder on activities of daily living? Look for a questionnaire that specifically targets these areas. A number of instruments are divided into domains-such as physical domain, social domain, emotional domain-that are the foci of groups of questions. You will also want to use an instrument that has been validated-that it measures what it purports to measure in the population of interest, that it can differentiate between groups with and without the condition of interest, and that it correlates with well-accepted existing measures of the same condition. You will certainly want an instrument that is reliable-one that can produce consistent results, and consistent results on different occasions, when there is no evidence of change.6 Finally, the ideal instrument will be responsive-it will be able to detect change over time, an important attribute if you wish to study treatment effects or the natural history of a disease. You may also wish to consider the burden of an assessment instrument, both for the patient and for staff members responsible for scoring the results: how long is it and how complicated; does it require special instructions in order to complete; is it available in languages other than English?
The burden of a questionnaire is often related to its format. An open-ended question would allow the respondent to give a free-text response of up to several sentences. This may result in more information being given, and the obvious disadvantage is that is requires considerably more time and skill to score, requiring subjective judgments. Closed-ended questions are more common and are used in most standardized measures. These include multiple-choice answers that permit one or several responses; the visual analog scale, in which the participant is asked to mark a line at a spot along a continuum from one extreme to another that best represents his or her experience; and the Likert scale, which is commonly used to quantify attitudes, behaviors, and domains of health-related quality of life.7 Closed-ended questions have the disadvantage of limiting the number of response options and leading the respondents in certain directions, unless there is the option to specify another response that is not on the list. However, the closed-ended question format is quicker and easier to answer and the answers are easier to analyze and to tabulate.
In the next column in this series we will discuss how to plan a prospective outcomes assessment in your practice setting using one or more of these instruments.
References
- 1. Bren L. The importance of patient-reported outcomes…it’s all about the patients. FDA Consumer Magazine, Nov-Dec 2006. www.fda.gov/fdac/features/2006/606_patients.html .
[Context Link] - Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Med Care 1992;30(6):473-83.
[Context Link] - Bergner M, Bobbitt RA, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981;19(8):787-805.
[Context Link] - Health Outcomes Institute. Health outcomes institute outcomes measuremement instrumentation. (Report No. Rev. 11/01/93). Bloomington, MN: Health Outcomes Institute, 1994.
[Context Link] - Radosevich DM, Werni TLK. A Practical Guidebook for Implementing, Analyzing, and Reporting Outcomes Measurements. Bloomington, MN: Stratis Health, 1997.
[Context Link] - Scientific Advisory Committee of the Medical Outcomes Trust: Assessing health status and quality of life instruments: attributes and review criteria. Quality of Life Research 2002;11:193-205.
[Context Link] - Hulley SB, Cummings SR, Browner WS, Grady DB, Newman TB. Designing Clinical Research (3rd ed.). Baltimore: Lippincott Williams & Wilkins, 2007.
[Context Link]
Laryngoscope Highlights
Anatomy-and Variations-of the Sphenopalatine Foramen
Although the success rate of sphenopalatine ligation is greater than 95%, there have been some reports of difficulties in isolating the arteries during endoscopic surgical procedures. Some anatomical variations on the nose lateral wall have been noted with reference to the location of the sphenopalatine foramen (SPF), the presence of an accessory foramen, artery ramification, and SPF dimension and morphology. Francini G. M. Pádua, MD, and Richard L. Voegels, MD, conducted a cadaver study to describe the anatomy of the SPF region and possible anatomical variations.
Bilateral dissections were performed on 61 cadavers that had no evidence of previous nasal trauma or sinonasal surgery. The investigators examined the ethmoidal crest of the perpendicular plate of the palatine bone; the location of the SPF, and the presence and location of the accessory foramen; distance from the SPF and accessory foramen to the anterior nasal spine; and the number of arterial branches emerging through the SPF and through the accessory foramen.
The researchers found that the ethmoidal crest was present in all cases; it was anterior to the SPF in 98.4% of cases. The most frequent (86.9%) location of the SPF was the transition area between the middle and superior meatus. The accessory foramen was present in only 9.83% of cases; in all but one of these, it was located within the middle meatus. The average distance from the SPF to the anterior nasal spine was 6.6 cm, and the average distance from the accessory foramen to the anterior nasal spine was 6.7 cm. Most nasal fossae (67.21%) presented only a single trunk, emerging through the SPF. In all cases, the accessory foramen presented only one branch. The researchers found no significant association of any variable with the presence of the accessory foramen.
The investigators note that the ethmoid crest, present in all cases, is an important anatomical landmark to find the SPF. Although trying to predict when an accessory foramen would be present could be of great value to minimize therapeutic failure, the prediction of its presence was not possible in relation to the variables analyzed in this study.
(Laryngoscope 2008;118:156-61)
©2008 The Triological Society
Leave a Reply