The incidence of thyroid cancer is increasing rapidly in the United States and the past few years have seen dramatic shifts in the management of papillary and follicular thyroid cancer. Knowledge of these emerging trends is important to optimize patient care and endocrine referral patterns for head and neck surgeons performing thyroid surgery.
Explore This Issue
December 2006We really need to look at where we stand in what I think is going to turn out to be a clinical epidemic, said Shereen Ezzat, MD, Professor of Endocrinology and Metabolism at the University of Toronto and a senior scientist in the Division of Applied Molecular Oncology at the Ontario Cancer Institute.
Dr. Ezzat was one of a distinguished roster of endocrine, imaging, and surgical experts on hand to update attendees on the most current trends and evolving paradigms in the diagnosis and management of thyroid cancer at the recent American Academy of Otolaryngology-Head and Neck Surgery annual meeting in Toronto.
It’s anticipated that, in the next five years, diagnoses of thyroid cancer are going to double, he said We have to look at the scope of the problem and where the deficiencies are-and, unfortunately, there are lots of them.
At the Molecular Level
Dr. Ezzat discussed the evolution of molecular diagnostic techniques and how those techniques are going to affect physicians’ understanding of the disease and, ultimately, how that will be translated into the therapeutic arena.
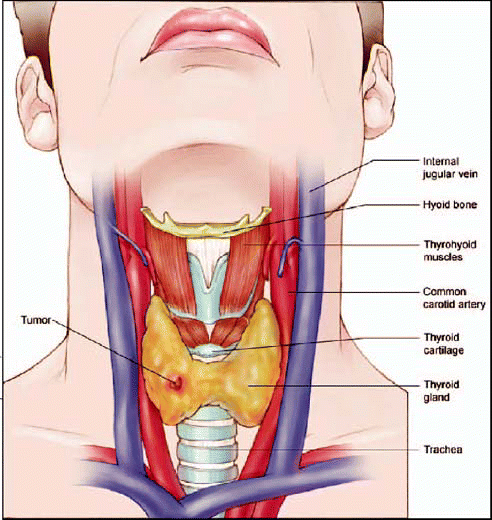
A thyroid nodule can be benign, it can be cystic, inflammatory, or it could represent an area of compensatory regeneration or hyperplasia, he said. But what we’re really most interested in is the area of neoplasia, where potentially clinical significance would have the greatest meaning.
Before subjecting a patient to diagnostic testing, as well as determining which tests to perform, Dr. Ezzat said physicians should consider the following statistics from a recent study: In nonirradiated patients, the risk of malignancy is 5%, while in irradiated patients, the risk increases to 30% to 40%. If there is some degree of autonomous function, the risk is less than 5%.
That’s what I already know, and if any test does not really add to this, then it’s kind of useless, he said. Part of the problem is that we like to rely on morphology. Morphology requires at least six criteria based on aspirate findings to make the diagnosis of papillary cancer. And only when all these criteria are met is the pathologist able to tell you that you’ve got papillary cancer. That’s the limitation, so I don’t think we should be too hard on the pathologist.
Dr. Ezzat believes a new paradigm is needed that explains how a thyroid follicular cell becomes either hydroplastic on its way to becoming adenomatous or sometimes turns into carcinoma.
This is the kind of paradigm people have used with breast and colorectal cancer, he said. Unfortunately, in thyroid cancer, we have not yet identified this kind of morphologic pattern. In other words, it appears that most of the nodules, by the time we pick them up, are already carcinoma, and very rarely do we see the evolution. Sometimes we see well-differentiated cancer turning into de-differentiated cancer, but typically what we start out with is cancer from the get-go. I think this is a bit of a problem in terms of understanding what defects really lead to this diagnosis.
Dr. Ezzat outlined some of the abnormalities that have been identified so far, emphasizing what he believes are some of the more important ones.
He said current research has shed some very important light on the area of chromosomal instability-where two genes become widely spaced, for example, and the normal regulatory sequences that drive these genes to become expressed become abnormal. Some of the hardiest information, he noted, is in the area of RET/PTC rearrangement.
We do know for sure that there is some degree of genomic instability where chromosomes do not maintain fidelity, Dr. Ezzat said. When they replicate they undergo some degree of instability that allows for rearrangements to occur, and the two most important ones are RET rearrangement and BRAF mutations. This is what takes you from a follicular cell to a papillary thyroid cancer.
At the cell surface, RET can be activated through either a rearrangement or an activating point mutation. As a result, it turns on a signaling cascade that sends information to the nucleus, turning on that cell to multiply and grow abnormally and autonomously.
This the type of rearrangement that reflects chromosomal instability, he said. It’s as if the genes no longer maintain the same kind of fidelity-molecular hanky-panky, if you will, whereby the RET is now being driven by an abnormal promoter, so it’s being revved up by something else. And it turns out that there is not just one type of rearrangement involving RET, but at least 15 or even more.
How is this type of genetic rearrangement detected?
You can do it on DNA, Dr. Ezzat said. You can also do it on RNA, which is obviously a little more difficult because you need a frozen sample for that or, in fact, you can do it at the protein level by immunohistochemistry. If you use immunoreactivity, then you’re able to identify the ectopic, abnormal expression of RET in the follicular cells.
BRAF has been identified as the most prevalent of the mutations identified in papillary thyroid cancer.
This kind of worries me, because if BRAF is a marker of aggressive behavior, then you would expect that a large majority of our patients from whom samples were obtained, are likely to go on to have bad aggressive disease clinically, and I’m not sure this is necessarily true, Dr. Ezzat said. I would emphasize that on the aspirates, you can do RET/PTC rearrangements and you can do BRAF point mutations. And if you do these two and combine it with morphology, at least as of 2006, you’ve got it pretty much nailed in terms of combining molecular diagnosis with morphology.
Dr. Ezzat said there are drugs available now that specifically inhibit BRAF and they have been shown in recent studies to actually inhibit tumor growth, indicating that if it is possible to target this pathway, it’s not just of diagnostic value, but potentially of therapeutic value.
What I’m worried about, however, is not so much the diagnosis of papillary cancer, but I’m worried about papillary cancer that is going to invade, potentially metastasize, and potentially have impact on morbidity and mortality in that patient, he said. I think that’s where our efforts really need to lie.
And it’s not just the thyroid cancer that researchers and clinicians need to be thinking about, Dr. Ezzat emphasized, but also the thyroid neighbors-the so-called stromal elements and the matrix outside.
It’s this neighborhood interaction between the cancer cell, the host, and its cellular environment that’s going to be absolutely crucial in teaching us how we’re going to stop cancer from being a small indolent malignancy to one that has the ability to invade and metastasize and cause a lot more clinically aggressive disease, he said.
Risk Stratification
Approximately 25,000 cases of thyroid cancer were diagnosed in the United States last year, with an expected 30,000 this year. Fortunately, most of the patients with thyroid cancer are going to do very well. But how do you identify those few patients who are going to do poorly?
We used to say thyroid cancer was a rare disease, but if you look at the actual number of thyroid cancer cases diagnosed, it’s really not much different than the number of Hodgkin’s disease, myeloma patients, and kidney cancers that are diagnosed throughout the United States, said R. Michael Tuttle, MD, an endocrinologist at Memorial Sloan-Kettering Cancer Center in New York. The numbers have actually more than doubled in women since the 1970s, from six per 100,000 women to 13 per 100,000, and those numbers are going to go up.
In order to manage those numbers, Dr. Tuttle emphasized the importance of detection of recurrent disease and persistent disease and the importance of risk stratification.
Risk stratification is much more than the argument about total thyroidectomy versus less than total, or yes or no on radioactive iodine, Dr. Tuttle said. Risk stratification is much more than that; it helps me understand which test to use.
Dr. Tuttle said the good old days are gone when people either had thyroid cancer or they didn’t.
Life was easy back then; their scan was either positive or negative, he said. And then we messed things up by developing thyroglobulin assays. We had all those people that we thought were cured of thyroid cancer and now, all of a sudden, we’re not so sure. If you look at the literature of the 1970s and 80s, this was a big puzzle. How could people have negative scans, but yet we’re measuring all this thyroglobulin?
The answer, he said, is that now researchers understand that thyroglobulin is a marker of persistent disease.
The ultrasound helped us out a lot because most of these patients had little low-level thyroglobulin after their total thyroidectomy and after radioactive iodine, Dr. Tuttle said. In the last 10 years, though, there has been a dramatic fall-away from radioactive iodine. When I was a fellow in the army, I was taught that I should talk people into doing radioactive iodine scans once a year for five years, with traditional hypothyroid withdrawal, taking their pills away. And, if you can talk somebody into going off their pills once a year for five years, we would like to hire you at Memorial to write grants for us, because you are a very persuasive surgeon.
Fortunately, he continued, over the past several years, there has been a big shift away from that approach.
In the setting of someone who’s had a total thyroidectomy and radioactive iodine, Dr. Tuttle said thyroglobulin should be measured every 6 to 12 months for the first year or two and then once a year after that. Ultrasound should be performed six months after surgery and again a year after surgery.
It’s not good enough to risk-stratify someone five minutes after surgery, he said. We are going to continually risk-stratify them based on time and all of the information that we have.
Another advantage of thyroglobulin measurement, Dr. Tuttle pointed out, is that it allows the physician to localize the disease.
If you call me and someone has a thyroglobulin of 1000 or 2000, ultrasound is not going to be my first choice, he said. Because when the thyroglobulin is that high, it almost always means distant metastasis, and the vast majority of the time, it’s metastatic bone disease somewhere. There’s something about bone, and the microenvironment in bone, that even small tumors produce remarkable levels of thyroglobulin. If, on the other hand, the thyroglobulin is in the 100 range, it’s probably lung metastasis. The serum thyroglobulin can point you in the right direction.
Imaging Thyroid Cancer
Another major paradigm shift has been the use of ultrasound, often in conjunction with other imaging modalities in the diagnosis, presurgical staging and postoperative management of thyroid cancer.
In the thyroid, only half of nodules even above 1 cm in size are palpable, noted Beth Edeiken, MD, of the University of Texas M. D. Anderson Cancer Center in Houston. In the case of nodules below 1 cm in size-which can be quite biologically aggressive tumors also-94% are nonpalpable and cannot be discovered by physical examination.
When you go to the lymph nodes of the neck, 30 to 50 percent of patients have lymph nodes that can’t be detected by physical examination, Dr. Edeiken said. That’s where ultrasound comes into play.
One problem with ultrasound, she noted, is that it cannot image the area behind the thyroid.
So, often we’ll see one or two lymph nodes that are abnormal in the central compartment, and then our surgeons will find four or five that are behind the thyroid, she said.
From personal experience I think it’s the better modality, if it’s done correctly, to look for recurrences in those deep, dark locations that your fingers can’t find and ultrasound can’t find, said Laurie A. Loevner, MD, a radiologist and neck cancer specialist at the University of Pennsylvania. In the setting of rising thyroglobulin, post-thyroidectomy, clinically negative exam in differentiated thyroid cancer, I would recommend that you start with an ultrasound. If ultrasound is negative, I would move on to an MRI or a CT, whatever you’re more comfortable with. Then you may do PET-CT when ultrasound and MRI are both negative.
Another one of the drawbacks of ultrasound, according to Dr. Tuttle, is the inherent and variable level of user dependency.
The trick with the neck ultrasound is who does it, he said. Because if it’s the same guy that’s doing your gall bladder ultrasound and your testicular ultrasound along with your post-op neck ultrasound, there’s a chance that you’re not going to get very great results.
An emerging trend that has more and more surgeons being trained and personally performing their patients’ imaging tests is encouraging, he added.
I think there’s no better person to do the neck ultrasound than the surgeon who is about to operate on, or has previously operated on, the patient, Dr. Tuttle said. Surgeons understand three-dimensional anatomy better than anyone on the planet. They understand the risks and benefits, so this trend toward surgeons learning to do ultrasonography, I think, is very good.
Managing Patients
Putting some of these new paradigms into practical terms, Dr. Tuttle offered his insight regarding the management of both low-risk and high-risk patients.
In low-risk patients-and by low risk I mean your 20- and 30-year-olds with papillary thyroid cancer, 2- or 3-cm papillaries, and two to four lymph nodes-the typical bread-and-butter 30-year-old womanwith thyroid cancer-the first thing that we do, down the road at six months or a year, is to measure thyroglobulin, he said. That’s our first test. It’s not a radioactive iodine scan; it’s not a PET scan and it’s not a CT. A physical exam and a serum thyroglobulin is where we start.
If, one year later, that patient still has measurable thyroglobulin, they either still have some normal thyroid tissue left in the neck or they have persistent disease and a surgeon should go in and find that disease, he said.
Unfortunately-and this is my truth in advertising, as much as it kills me to admit it-thyroid cancer is a surgical disease, Dr. Tuttle said. If at all possible, if there are lymph nodes, if there is disease remaining, it should be surgically excised. That’s the best way to treat this.
Ultrasound is the first stop, he said, for the patient with positive thyroglobulin.
It makes sense to do the ultrasound first, see if they have surgically correctable disease; it’s reasonable to also do a chest X-ray and then move toward radioactive iodine, he said. Fortunately for most patients, a year down the road, the thyroglobulin is undetectable or almost undetectable.
That measurement, however, is potentially unreliable, Dr. Tuttle cautioned, because about 20% of these patients who have an undetectable thyroglobulin at one year will still measure positive for thyroglobulin when stimulated, suggesting that some disease is still present.
When a paper on this came out, I started stimulating these patients. I thought I was trying to find these people with low-level thyroid cancer, so that I could cure them with more radioactive iodine or more surgery, he said. It turns out, though, that’s not the reason to do this test. The reason is to find those patients that were low-risk patients who, a year down the road, have an undetectable thyroglobulin and when you stimulate them, they still have an undetectable stimulated thyroglobulin. This is as close to a cure in thyroid cancer as we’re ever going to get.
Obviously, there are going to be some recurrences, he noted, and there will be some small lymph nodes, so these patients do need to be followed, but they get taken out of an intensive follow-up program and put on once-a-year program.
This is a nice paradigm to use, he said. It allows us to identify patients that are probably cured of their disease.
Diagnosis Tips from the Experts
Why is fine-needle aspiration so uncertain? Because a great number of cells are needed to be able to look at the morphology of the nucleus as well as the cytoplasm.
In about 20 percent of cases, there is simply not enough material, and another 20 to 30 percent where the material is of an indeterminate nature, he said. This means in at least 40 percent of cases, you’re going to have unanswered questions. The big problem is that nobody wants to come back to a patient five years later and tell them that nodule was really of significance and you missed it. – Shereen Ezzat, MD
On the shift away from radioactive iodine:
It’s not that I’m getting rid of nuclear medicine, and it’s not that I’d never do radioactive iodine scans, but they are no longer the primary tools that we use. Our primary tool really revolves around the thyroglobulin blood test and now, most importantly, neck ultrasound. – R. Michael Tuttle, MD
Why do ultrasound?
We know that ultrasound is not the most elegant imaging technique; people like PET and CT. But ultrasound of the cervical nodes makes a major contribution to the presurgical planning of thyroid cancer by detecting early ipsilateral and contralateral clinically occult cervical node metastasis that otherwise would not have been included in the surgical dissection. – Beth Edeiken, MD
Send us your Feedback
We’d like to know what you think about our articles. Please feel free to respond to our stories by e-mailing ENToday@wolterskluwer.com. When writing in, please include your full name, title, phone number, and e-mail address.
©2006 The Triological Society
Leave a Reply