ORLANDO-Concurrent chemoradiotherapy (CRT) is considered standard treatment for unresectable advanced head and neck cancer. A new study presented at the 2009 meeting of the American Society of Clinical Oncology (ASCO) suggests that induction chemotherapy (IC) delivered before CRT significantly improved time to treatment failure (TTF) compared with standard upfront CRT alone in this setting.
Explore This Issue
August 2009Induction chemotherapy prior to CRT is feasible and achievable. The sequential strategy doubles TTF and achieves a significant benefit in locoregional control. Induction chemotherapy should be considered the new standard of care, said Ricardo Hitt, MD, of the University Hospital 12 de Octubre in Madrid.
Although this presentation was selected for inclusion in the Best of ASCO program (an educational initiative that highlights important presentations from the ASCO annual meeting), the formal discussant of the trial took issue with aspects of the design and told listeners that more research is needed before induction chemotherapy can be considered a standard.
Study Details
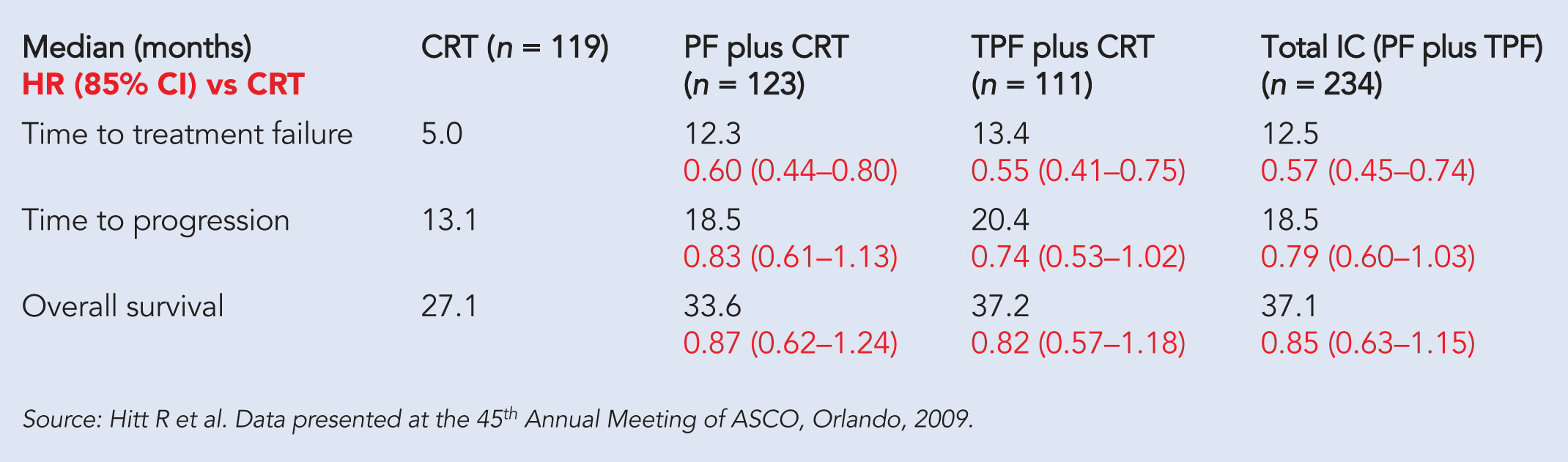
The study enrolled 439 patients with locally advanced head and neck cancer with good performance status. Patients were randomized to receive standard CRT with cisplatin and fractionated radiation versus CRT preceded by IC (sequential approach). TTF was defined as time from randomization to progression, recurrence, surgery, death, or no locoregional control. PF (cisplatin plus 5-fluorouracil) and TPF (docetaxel plus cisplatin plus 5-fluorouracil) were given in three cycles every three weeks and were followed by CRT.
At a median follow-up of 37.6 months, sequential therapy improved TTF from five months with CRT to 12.5 months (P < 0.0001), representing an absolute increase of 7.5 months. Time to treatment progression (TTP) was a median of 18.5 months in the sequential arm versus 13 months in the CRT arm. The sequential arm improved the secondary endpoint of locoregional control from 44.5% in the CRT-alone arm to 61.5% (P = 0.002). Patients treated with IC plus CRT had a median overall survival of 37 months versus a median of 27 months for CRT alone (see table).
Most of the treatment failures in the trial were due to locoregional recurrence, he noted.
Stomatitis and febrile neutropenia were the most frequent grade 3 and 4 adverse events. Grade 3 and 4 stomatitis occurred in 44% of IC + CRT versus 31% for CRT; grade 3 and 4 febrile neutropenia occurred in 10% and 1%, respectively. Other adverse events included neutropenia and asthenia.
During the discussion session following his presentation, Dr. Hitt said that patients in the IC arms had considerable toxicity and comorbidity until GM-CSF was added along with chemotherapy. These patients need supportive care, he said.
 Leaving out 25% of the patients is a serious problem. In my view, this is not a new standard based on study results.
Leaving out 25% of the patients is a serious problem. In my view, this is not a new standard based on study results.
-Jan B. Vermorken, MD, PhD
Questions Raised
The formal discussant of the trial, Jan B. Vermorken, MD, PhD, of University Hospital in Antwerp, said that this study raised many questions and, in his view, the sequential arm should not be considered a new standard based on results of this trial.
He took issue with the uneven distribution of patients in the trial: 128 in the CRT arm, 156 in the PF arm, and 155 in the TPF arm. He also noted that there were 11 treatment-related deaths in the sequential arm and only two in the concurrent CRT arm.
The summary of efficacy was most disturbing, he said. Eighty-six patients were left out of the analysis [nine in the CRT arm, 33 in the PF arm, and 44 in the TPF arm]. Leaving out 25% of the patients is a serious problem. In my view, this is not a new standard based on study results, said Dr. Vermorken.
©2009 The Triological Society
Leave a Reply