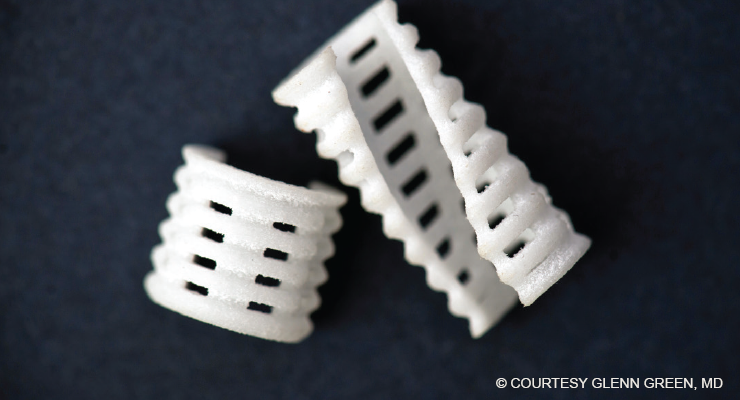
Fig. 1. A resorbable trachea stent used in the treatment of tracheobronchomalacia in infants.
Three-dimensional (3D) printing is transforming the field of pediatric otolaryngology. Nine years after implanting the first 3D bioabsorbable tracheal splint in an infant boy with severe tracheobronchomalacia (N Engl J Med. 2013;368:2043-2045), a multidisciplinary team of surgeons and biomedical engineers has successfully created a neotrachea supported by a 3D-printed scaffold to treat an infant born with tracheal agenesis, a rare congenital anomaly that’s usually fatal.
Explore This Issue
February 2021“3D printing is allowing us to treat diseases that weren’t treatable before,” said Glenn Green, MD, professor of otolaryngology–head and neck surgery at the University of Michigan Medical School, Ann Arbor, and one of the lead surgeons involved in both cases. “It’s opening up new ways of making devices that are specific for individuals who have rare conditions who, if left untreated, would not survive.” The infant was the first child to undergo this procedure; she’s doing well and is already the second-longest surviving child born with this condition in the United States, Dr. Green noted.
Tracheal Stent
Although there are many surgical strategies for managing tracheobronchomalacia, such as tracheostomy, tracheal resection, and slide tracheoplasty, before the advent of the 3D approach, surgeons were running out of options for treating very ill infants. The idea for creating a 3D trachea stent was born out of a conversation Dr. Green had with a colleague. “I was talking about how we had these kids whom we were unable to treat. I was introduced to Scott Hollister, PhD, an engineer, and together we developed something that has helped a lot of children.” Dr. Hollister is now a bioengineer in the Wallace H. Coulter Department of Biomedical Engineering at the Georgia Institute of Technology in Atlanta.
First implanted in 2012, the 3D-printed scaffold stent is designed to go around the outside of the diseased portion of the trachea to allow air to freely flow into the lungs (Fig. 1). To date, Dr. Green and his colleagues at the University of Michigan have treated 34 patients using the device. “We’ve taken individuals who were unable to leave the intensive care unit for months due to serious episodes of respiratory obstruction, and they’re now home with their parents. These conditions are now very treatable,” Dr. Green said.
Because the implant is porous, it creates a conductive space for blood vessels to grow. Within the first couple of weeks, the patient’s own blood vessels quickly grow into the scaffolding. —David A. Zopf, MD, MS
One of the main benefits of the device is that it’s made of a resorbable material designed to dissolve after approximately three to four years. As the tissue grows into the stent, it’s reconfigured to the shape of the scaffold. “By reconfiguring the way that the tissue grows, over time the original problem goes away,” said Dr. Green. “We now have about nine years of follow-up, and our early patients are growing and thriving.”
Dr. Green and his University of Michigan colleagues are in the process of applying for FDA approval for the splint procedure so that it can be made available to a wider group of surgeons and patients.
Multiple Benefits of 3D Printing
The application of 3D printing in otolaryngology falls into four broad categories: presurgical models, educational models for training, medical devices, and tissue engineering, according to Robert J. Morrison, MD, assistant professor in the department of otolaryngology–head and neck surgery at the University of Michigan. Early on, 3D printers were used to construct surgical models for preoperative planning and intraoperative guides; these models can also be used as educational models for surgical simulation (see “3D Simulators as Training Tools” on page 12).
None of these intricate surgeries would be possible without the field of tissue engineering. Tissue engineering falls into two categories, Dr. Morrison noted: scaffold-based and bioprinting. In the scaffold-based approach, cells are seeded into the scaffolding. As the scaffold gradually dissolves, “you’re left with a structure that’s made out of that native tissue in the desired 3D shape that you want,” Dr. Morrison said.
The advantage of 3D printing is the ability to customize the stent for each individual patient and avoid many of the pitfalls of traditional metal and silicone stents. “3D printing has become nearly synonymous with patient-specific manufacturing,” said Joshua A. Stramiello, MD, a resident in the division of otolaryngology–head and neck surgery, department of surgery, University of California, San Diego.
In a review of 3D-printed stent procedures in children, Dr. Stramiello and his colleagues found that, “given recent improvements in materials science, there is a growing body of evidence suggesting a strong role for bioresorbable intraluminal stents in treating pediatric tracheobronchial obstruction” (Int J Pediatr Otorhinolaryngol. 2020;132:109923).
These procedures aren’t without risks, however. In a pooled analysis of pediatric trials of scaffold-based procedures using intraluminal polydioxanone (PDO) stents, Dr. Stramiello and his colleagues reported an overall complication rate of 21.7%, with a stent fragment foreign body being the most common (8.7%), followed by significant granulation tissue (4.3%), stent migration (4.3%), and local stenosis (4.3%) (Int J Pediatr Otorhinolaryngol. 2020;139:110405). Dr. Stramiello noted that although comparative animal studies report biocompatibility and fewer morbidities compared with metal and silicone stents, in human studies, there are concerns over the short interval of degradation and the potential for obstructive foreign bodies in poorly seated stents.
[3D printing is] opening up new ways of making devices that are specific for individuals who have rare conditions who, if left untreated, would not survive. —Glenn Green, MD
“Fortunately, the treatment success using the 3D-printed airway device [polycaprolactone] has just been miraculous,” said David A. Zopf, MD, MS, assistant professor in the department of otolaryngology–head and neck surgery, University of Michigan, who was part of the team that conducted the first tracheal splint procedure. “Despite this being used in critically ill patients who have not responded to standard treatments, the number of complications has been exceedingly rare,” he said (Laryngoscope. 2019;129:1763-1771).