Affecting more than 30 million Americans, chronic rhinosinusitis (CRS) has been a frustrating disease with no long-lasting results from traditional steroidal and antibiotic treatment, or from surgery. With both clinicians and patients desperate for a solution, it is not surprising that hope-and controversy-has arisen over a potential new therapy.
Explore This Issue
March 2008The use of the antifungal agent amphotericin B to treat noninvasive fungi in CRS has become one of otolaryngology’s hot-button issues. Even though the National Institutes of Health (NIH) announced in a 2004 press release that people with chronic sinus inflammation have an exaggerated immune response to common airborne fungi, many in the medical community are hesitant to embrace an antifungal, one-size-fits-all treatment for all CRS patients.
The concept is that airborne fungi gather in the nose and sinuses and are attacked by eosinophils, which produce secondary mucosal inflammation in susceptible individuals. The debate regards the link between these fungi and CRS. Most specialists regard allergic fungal sinusitis (AFS) as a subset of chronic rhinosinusitis (CRS), which has been defined as any inflammatory disease of the nose and paranasal sinuses that lasts longer than three months. Traditionally, the opinion was that AFS consists of fungus within the sinuses and allergy to that fungus. However, studies in recent years suggest that conventional allergy does not matter-that the immune response to fungi may be mediated through a lymphocyte-dependent mechanism independent of an immunoglobulin E (IgE)-mediated allergy.
The issue of whether fungi causes all CRS gained national attention nearly a decade ago when researchers at the Mayo Clinic announced that fungi were present in nearly all CRS patients, as well as in healthy individuals. After publication of their first data in Mayo Clinic Proceedings (1999;74:877-84), the team demonstrated in follow-up studies that intranasal antifungal treatment (specifically with amphotericin B) improved the objective computed tomography (CT) findings such as inflammatory mucosal thickening, nasal endoscopy stages, and CRS symptoms. After several noncontrolled studies were published, the team further demonstrated positive results with a randomized, double-blind, placebo-controlled trial of 30 CRS patients (J Allergy Clin Immunol 2005;115:125-31).
Studies by researchers in Switzerland and Italy have supported the Mayo findings. Ricchetti et al. (J Laryngol Otol 2002;116:261-3) stated that hyper-reactivity to fungal organisms should be one of the mechanisms underlying the development of nasal polyposis. Additionally, the researchers stated that amphotericin B seems to induce the disappearance of nasal polyps in about 40% of patients.
In 2006, Italian researchers (Corradini et al., J Investig Allergol Clin Immunol 2006;16(3):188-93) said their study indicated that long-term topical treatment with lysine acetylsalicylate and amphotericin B may be clinically effective in the treatment of patients with nasal polyposis associated with fungal infection.
As a result of these studies, Accentia BioPharmaceuticals, Inc., obtained a license from the Mayo Clinic to develop the amphotericin B treatment under the name SinuNase™. With an investigational new drug application, Accentia received fast track designation from the US Food and Drug Administration to test the treatment in clinical trials. Results will be unblinded this month.
The Critics Are Skeptical
Meanwhile, critics of the fungal theory worry that practitioners might adopt use of an antifungal agent when only limited data are available to prove effectiveness. Others point to studies by two separate European groups that found that amphotericin B was ineffective in CRS treatment.
The issue is whether this is a universally effective or even appropriate treatment for all forms of CRS, said Bradley F. Marple, MD, Professor of Otolaryngology at University of Texas Southwestern Medical School in Dallas. Unfortunately, we have not yet fully characterized the pathogenesis of CRS. Based on the limited evidence that is currently available, many feel that amphotericin B may be appropriate for a subset of patients with CRS, but not all patients. At present, there are several attractive nonfungal etiologies under investigation that may also act as underlying triggers of CRS, such as bacterial superantigens, biofilms, and allergies. These are all things that could work independently or in conjunction with one another to drive the inflammatory process.
Dr. Marple further said that he was worried about an idiosyncrasy in the Mayo Clinic study. Although the Mayo team reported a lessening of mucosal thickening, there was no change in intranasal Alternaria and inflammatory cytokines failed to change in a statistically significant fashion. The fact that nasal carriage of Alternaria was not affected by this therapy appears to challenge the underlying hypothesis that inflammation is actually driven by fungus, he said.
Michael Weschta, MD, from the University of Ulm, Germany, is one of the European researchers who believes that amphotericin B shows no evidence of clinical improvement in CRS patients. His team used a randomized, double-blind, placebo-controlled trial with 78 CRS patients and found no significant benefits from eight weeks of amphotericin B nasal spray therapy (J Allergy Clin Immunol 2004;113:1122-8). The Weschta team further reported in a 2006 study that neither topical amphotericin B therapy nor fungal state before and after treatment had any significant influence on activation markers of nasal inflammatory cells in chronic rhinosinusitis (Arch Otolaryngol Head Neck Surg 2006;132:743-7).
Fenna Ebbens, MD, from the Academic Medical Center in Amsterdam, found in 2006 that treating 116 CRS patients with amphotericin B nasal lavage or placebo failed to show improvement in symptoms, nasal endoscopy scores, and other markers (J Allergy Clin Immunol 2006;118:1149-56). In a 2007 review article in Rhinology (45:178-89), she further stated that we conclude, on the basis of the results of our large, double-blind, placebo-controlled, multicenter study that direct topical administration of intranasal amphotericin B is not a solution for patients with CRS with or without nasal polyps, because neither major improvements nor significant differences between amphotericin B-treated and placebo-treated groups were observed.
Dr. Ebbens told ENT Today that amphotericin B remains a valuable antimycotic systemic treatment for potentially life-threatening invasive mycoses. Presently, in the absence of convincing evidence on clinical improvement of CRS upon therapy with both topical and oral antifungal agents, we should be careful about advocating widespread use of this drug. Widespread use may lead to resistance and, in time, we may lose a valuable antimycotic systemic drug, which still demonstrates low resistance.
Which brings the debate back to the Mayo results and advocates of amphotericin B. Jens Ponikau, MD, the physician-researcher who brought amphotericin B treatment to national attention when he was a graduate student, then a researcher at the Mayo Clinic, continues his work today as Clinical Assistant Professor of Otolaryngology at the University at Buffalo, State University of New York. In a number of studies and reviews (including US Respiratory Disease 2007, Touch Briefings; Therapeutics and Clinical Risk Management 2007;3:319-25; Clin Rev Allergy Immunol 2006;30:187-94), Dr. Ponikau has explained his findings in recent years, which include an identification of the specific antigen (the fungal agent Alternaria), the substance it secretes (eosinophilic major basic protein, eMBP), and the manner in which it binds to inflammatory cells.
The Devil Is in the Details
Regarding studies that have contradicted his findings, Dr. Ponikau told ENT Today that the devil is in the details. Although study results may vary, I think the difference is how the studies were conducted, he said.
Noting that both the Weschta and Ebbens studies excluded patients with fungal etiology, he compared this approach to testing an allergy medicine and excluding everybody who had allergies. Additionally, the Weschta trial included only patients with severe nasal polyposis. Dr. Ponikau said the drug was probably unable to reach the sinuses and the majority of the nasal cavity, due to blockage from the excessive polyps, specifically when a spray was used.
He said that both studies also used drug formulations (putting the drug in glucose) that changed the osmotic pressure gradient. In order for the drug to work, it has to penetrate into the mucus, he said. By putting the drug into glucose, they reduced the osmolaric pressure gradient between the amphotericin B solution and the mucus, to the effect that lesser amphotericin B diffuses in.
Dr. Ebbens responded by pointing out a study by Kintzel et al. (Am J Hosp Pharm 1992;49:1156-64) that adding glucose instead of water to the solution reduced nasal irritation because of low osmolarity and has no effect on drug bioavailability. She also said that the addition of glucose is advised as diluent by the manufacturer. No proof exists that a pressure gradient is needed for treatment effect.
Also pointing out flaws in the Weschta and Ebbens studies was Francis E. O’Donnell Jr., MD, Chairman and Chief Executive Officer of Accentia BioPharmaceuticals, who said the limitation in the Weschta study is that he used a pump spray, not a lavage. And, he used a very high concentration of amphotericin B, 30 times the concentration that Mayo and we used in our clinical trials. He used 3 milligrams per cc and anything above 500 micrograms, or half a milligram per cc, is likely to be toxic to the epithelia.
Both Dr. Ponikau, who is not affiliated with Accentia, and Dr. O’Donnell also criticized Dr. Ebbens’s use of a gravity-fed drug delivery device that was designed to deliver 250 cc. However, in their trials, it was only filled with 25 cc-indicating that the pressure may have been too low to effectively administer the drug. Dr. O’Donnell noted that SinuNase, in contrast, is delivered with the equivalent of a bulb syringe so patients can apply sufficient pressure to deliver it throughout the nasal cavity.
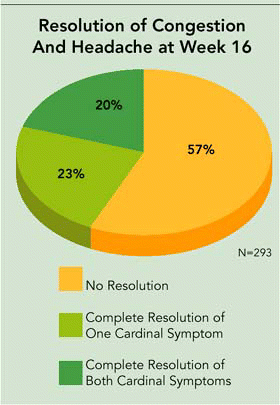
Dr. O’Donnell was enthusiastic about his own company’s current Phase III clinical trials of SinuNase. He said that Accentia expects to present the unblinded Phase III clinical trial primary results in mid-March. These will include complete resolution of the cardinal symptoms of nasal congestion and sinus headache/facial pressure. The secondary endpoints, including endoscopy and CT scan results, will be available at a later point and presented at a scientific forum.
In January of this year, Dr. O’Donnell announced in a news release and letter to shareholders that we now have interim, blinded, intent-to-treat data on the primary endpoint (complete resolution of both cardinal symptoms) at the conclusion of the study for approximately 80% of the patients in the study. This interim blinded data shows that approximately 20% of all patients are achieving the primary endpoint of complete resolution of both cardinal symptoms and another 23% of patients are achieving complete resolution of one or the other cardinal symptom at 16 weeks. To put these results in perspective, it is important to remember that 50% of the patients received SinuNase and that 50% received a placebo control that had no antifungal activity.
We think there are several implications of this study, Dr. O’Donnell told ENT Today. Assuming that unblinding confirms our interpretation of the blinded data, a fungal-induced inflammation as the cause of chronic sinusitis is a valid paradigm. It’s a host-defined disease where only 10 percent of those exposed to fungi mount an inflammatory response characterized by eosinophils.
He added that Mayo was right. A topical intranasal administration of extremely low doses of antifungals looks like it will be significantly effective at reducing the symptoms and inflammatory changes in the nose and in the sinuses. The clinical trial is suggesting that the vast majority of bona fide CRS cases are due to the fungal-induced inflammation. Although patients in the Phase III study were not selected on the basis of whether or not they had nasal mucin that was positive for eMBP, after enrollment, every patient has tested positive so far for eMBP.
Clearly, the results are incompatible with what was shown by Weschta and Ebbens, Dr. O’Donnell further stated, noting that the studies by the European researchers showed no effect on symptoms or signs, whereas the Accentia trial with 302 patients indicates at the still-blinded point that roughly half of the patients have a significant resolution-not just a reduction in severity-of symptoms and improvement in endoscopy and CT scans of the sinuses.
Dr. Ebbens cautioned, however, that the Accentia results may reflect a placebo effect. Before drawing any conclusions, we should await the unblinded results, she said.
This is the first time that anything for CRS has made it into an FDA-approved Phase III trial, Dr. Ponikau said. No drug or treatment has ever gone that far for CRS. We are careful in what we are saying; we’re saying it’s in development and we’re learning a lot about it, but we’re not there yet.
A lot of patients are going to benefit from the research at the Mayo Clinic, Dr. O’Donnell added. But that research met with incredible skepticism because many people failed to understand what Mayo was saying. They weren’t saying that if you had the fungus, you had the disease. Rather, they were saying it’s a host-defined disease. Virtually everyone has the fungus in the mucus of their nose and sinus. But only 10 percent of the population has an inflammatory response to it.
Maybe Not for Everyone
Neil Bhattacharyya, MD, Associate Professor of Otolaryngology at Brigham and Women’s Hospital and Harvard Medical School in Boston, would agree, but with a caveat. He said, Amphotericin B is one of the members of our armamentarium that we use to treat chronic rhinosinusitis-in certain cases. I think it has a role in the treatment of a certain subset of CRS, but it’s not, in my opinion, going to be the end-all, be-all answer for why those patients don’t do well with traditional therapy or surgery.
Dr. Bhattacharyya further said that the real reason amphotericin B is controversial is because there’s a lot more to be learned about the pathophysiology and the mechanism of action of amphotericin B. Also, the underlying evidence for fungi as the cause-complete for CRS is actually weak. If you look at detailed studies, you can find people who have evidence of an allergic sensitivity to the fungus, but no symptoms.
Another component, he said, is that there is evidence that amphotericin B as a chemical agent alone is an anti-inflammatory, meaning that it may be working on the inflammation in chronic rhinosinusitis but not killing fungus.
On the plus side, Dr. Bhattacharyya said amphotericin B is a relatively low-toxicity agent when applied topically. I see little downside in trying this as one modality of therapy for patients who are medically refractory, he said, adding that it might be a good choice for patients who have already had surgery (surgery that allows the drug to reach the surface of the sinus cavities more directly). I do have a subset of patients who have benefited from it. But, I’ve also had patients who have not.
About Amphotericin B
An antifungal treatment-specifically, amphotericin B-was chosen by the Mayo Clinic and later tested by others for efficacy in treating CRS. Originally extracted from the filamentous bacterium Streptomyces nodosus, amphotericin B is used to kill fungus that can cause serious or life-threatening infections, but it is not effective against bacterial infections or viruses. Although administration of the drug can cause side effects-some potentially severe-the small amount used as a lavage to kill noninvasive fungus in the nose and sinus cavities is considered much safer. It has low systemic absorption, with less than 5% systemic absorption across mucous membranes. As its mechanism of action, amphotericin B binds to sterols-preferentially to the primary fungal cell membrane sterol, ergosterol-disrupting osmotic integrity of the fungal membrane. The result is leakage of intracellular potassium, magnesium, sugars, and metabolites, followed by cellular death.
©2008 The Triological Society

Leave a Reply