Recent studies indicate that the Bravo pH monitoring system from Medtronic may offer a safe and effective diagnostic tool for pediatric patients suffering from symptoms of gastroesophageal reflux disease (GERD). The visible catheters and discomfort associated with traditional diagnostic studies, along with the clinical setting required for some tests, often cause anxiety in adult patients, and can be downright frightening to a child. The Bravo system may offer a more comfortable and convenient testing option.
Explore This Issue
April 2006Diagnostic Gold Standard
Testing for the pH level is the gold standard for diagnosing GERD. Esophageal pH testing measures how often stomach acid flows into the lower esophagus, and the amount and degree of acidity during the test period.
Traditional pH testing involves placing a catheter through the patient’s nose and down the esophagus. This can cause the patient significant nasal and throat irritation, impairing the ability to maintain a normal diet. The catheter itself remains visible, causing many patients to alter their exercise and activity routines during the test period. These changes in the patient’s routine may provide false negative findings and adversely affect the reliability of the results.
The Bravo system may also provide data more representative of the patient’s day-to-day activities than traditional pH monitoring methods because patients are able to maintain a normal diet and activity level during testing.
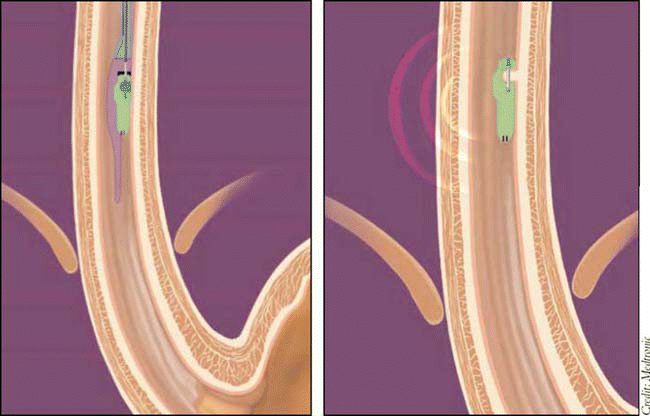
How the System Works
The Bravo system includes a capsule measuring 6 mm by 5.5 mm by 25 mm and containing a 3.5 mm deep well connected to an external vacuum unit. The physician inserts the capsule orally or transnasally into the esophagus during upper endoscopy. Using the vacuum unit, the physician pulls esophageal tissue into the well and literally pins the capsule into place. The delivery system is withdrawn, and the capsule is left in place to record pH data. Placement of the capsule takes about 10 minutes, and patient discomfort is minimal. Attachment of the capsule creates less trauma to the esophagus than a standard biopsy, and the point of attachment heals within several days.
Once the capsule is in place in the esophagus, it begins transmitting data via radio frequency telemetry to the Bravo receiver, which the patient wears on his or her belt. Once the test is complete, data are uploaded from the receiver to a computer for analysis and evaluation by a nurse or physician.
Because the patient cannot see or feel the capsule, he or she is able to maintain normal diet and activity during the test period. Patients (or parents) keep a diary recording food intake, symptoms, and activity, including position changes. The information recorded in the diary is then synthesized with the pH readings recorded by the system receiver.
The Bravo capsule detaches itself from the esophagus through a combination of natural tissue regeneration and sheer force from eating, generally within five to seven days.
Possible Complications
In a 2004 study on the use of the Bravo capsule for pH monitoring in adult patients, the probe did not attach properly in seven of 60 subjects (12%). In all but one patient, a replacement probe was attached without difficulty (Aliment Pharmacol Ther. 2004;19(4):449-454).
The potential exists for the capsule to fail to detach promptly, but according to the manufacturer there have been no reports of this actually happening to date. In the event that the capsule does not separate in a timely fashion, it can be seen on x-ray and removed endoscopically if necessary.
Researchers also warn that the physician must be careful to place the capsule below the cricopharyngeal muscle sphincter to avoid the possibility of the Bravo capsule creating a foreign body airway obstruction when it does disconnect.
Endoscopy Adds to Risk
Other potential complications are those associated with the procedures used for placement of the capsule. Potential adverse effects associated with nasal intubation for transnasal placement, which is used for both Bravo and traditional catheter placement, can include sore throat, trauma to the nasopharynx, or bloody nose.
In addition, since upper gastrointestinal endoscopy is used to ensure proper placement of the capsule, possible complications from that procedure must be considered as well and can include:
- Perforation
- Hemorrhage
- Aspiration
- Fever
- Infection
- Hypertension
- Respiratory arrest
- Cardiac arrhythmia or arrest
Except for painful symptoms, primarily sore throat, none of the patients or parents participating in published studies on the use of the Bravo capsule in pediatric patients have reported subjective complaints. In a study published in the Journal of Pediatric Gastroenterology & Nutrition, 94.7% of parents stated a willingness to allow their children to undergo wireless pH monitoring in the future (2005;41(4):411-415). Additionally, 12 subjects (out of 44), who had previously undergone nasal catheter pH probes, stated a preference for the wireless pH monitoring.
Marcella Bothwell, MD, assistant professor of otolaryngology and child health at the University of Missouri, uses the Bravo system in her clinical practice, but cautions that its use may not always be indicated, and additional diagnostic procedures may be necessary. The Bravo system is designed to monitor pH levels and does not detect other possible contributing factors to GERD, such as motility and pressure problems. Regarding Bravo, Dr. Bothwell said, “It is an adjunct to other methods of detection. The most important thing is suspicion of reflux. I still use other methods when I am not planning to do an airway endoscopy.”
Increased Cost
Even with the potential benefits in comfort and convenience offered by the Bravo capsule, some researchers question whether the test may be cost-prohibitive to the point of outweighing the potential benefits. List price of the Bravo pH monitoring system is $225, compared with about $62 for a traditional transnasal pH catheter. The addition of upper endoscopy for placement of the Bravo capsule also significantly increases the cost of the evaluation. In all, total cost of pH testing using the Bravo capsule is more than five times the cost of conventional pH probe testing.
Early Diagnosis of GERD Important
There is an increasing awareness that GERD may have its origin in childhood. If left untreated, GERD can lead to dysphagia, odynophagia, stricture, Barrett’s esophagus, chronic hoarseness or laryngitis, respiratory problems (such as coughing or asthma) or non-cardiac chest pain.
Studies have found that up to 75 percent of patients with symptoms of upper or lower respiratory tract disorders, such as asthma, croup, bronchitis, pneumonia, sinusitis, laryngomalacia and subglottic stenosis have reflux disease (Laryngoscope. 2004;114(4):786-788). Reflux must be diagnosed and treated in these cases to allow for proper respiratory treatment.
If GERD is diagnosed and treated in children, it may result in better long-term outcomes, such as improved quality of life and reduction in the overall health care burden. However, because GERD symptomatology is often atypical in children, and because traditional catheter-based monitoring is uncomfortable and cumbersome, many parents may forego testing for their children, leaving them undiagnosed, and consequently untreated, for this disorder.
Devices like the Bravo pH monitoring system may offer a more desirable diagnostic option for pH monitoring for pediatric patients and their parents in terms of comfort and convenience.
©2006 The Triological Society

Leave a Reply