A new, minimally invasive technology known as balloon sinuplasty can safely and successfully dilate blocked sinus ostia in select patients with chronic sinusitis, according to early observations in a small number of patients.
Explore This Issue
May 2006Researchers agree that the results of larger, longer studies are needed before anyone will know whether the technology-which involves threading a small, flexible balloon-tipped catheter through the sinus passageways and expanding the balloon to gently dilate the openings of the sinuses-will change standard treatment of chronic sinusitis.
Gentle Dilation, Less Trauma
But early studies suggest that the technology is less invasive than conventional endoscopic techniques, said William E. Bolger, MD, a rhinologist and sinus surgeon based in Bethesda, Md. Dr. Bolger headed the preclinical and pilot safety and feasibility studies that led to the recent Food and Drug Administration approval of the technology, known as the Relieva Balloon Sinuplasty System.
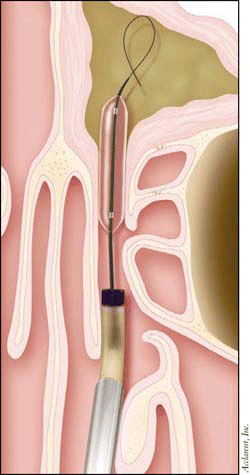
The device appears to impart less trauma, while preserving normal anatomy and mucosal tissue, he said. Less trauma during surgery tends to be associated with less bleeding, less pain, and perhaps less need for deep anesthesia. Recovery time could also be shorter, although more clinical testing is needed.
Andrew N. Goldberg, MD, Associate Professor in the Department of Otolaryngology-Head and Neck Surgery at the University of California, San Francisco, agreed. The rationale for the device is that it gently dilates the sinus ostia to allow for better ventilation and drainage. There is minimal damage to the mucosa and minimal bleeding and trauma as compared to standard endoscopic sinus surgical techniques.
The device appears to be particularly helpful for the frontal sinuses where it provides gentle dilation of the outflow tract with remodeling of adjacent cells, added Dr. Goldberg, who is participating in postmarketing studies of the device.
How it Works
The technology works much like balloon angioplasty to open up fat-clogged coronary arteries. First, the catheter is introduced into the target sinus under endoscopic visualization. A flexible guidewire is introduced through the catheter and gently advanced into the target sinus under fluoroscopic guidance. Then, a balloon-tipped catheter is positioned across the blocked ostium, with its position confirmed using fluoroscopy. The balloon is gradually inflated to gently restructure the blocked ostium. The system is removed, leaving the ostium open and allowing the return of normal sinus drainage and function.
The sinuses contain very small bones, as thin as an eggshell, Dr. Bolger explained. So when the balloon expands, microfractures are created and are pushed out of the way, leaving the sinuses open and restoring normal sinus drainage and function.
In the preclinical work on human cadavers, feasibility was demonstrated through 100% dilation of the target sinuses, he said. Importantly, comparison of pre- and post-CT scans, multi-angled endoscopic views, and gross examination showed the technology did not cause damage to surrounding structures such as the orbit or skull base. It did what we expected and did not cause any harm, he said.
Early Results Promising
A follow-up pilot study of 10 human patients with 18 blocked sinuses confirmed the safety of the technology, with the balloon catheter successfully dilating the ostium in all patients without any complications or adverse events.
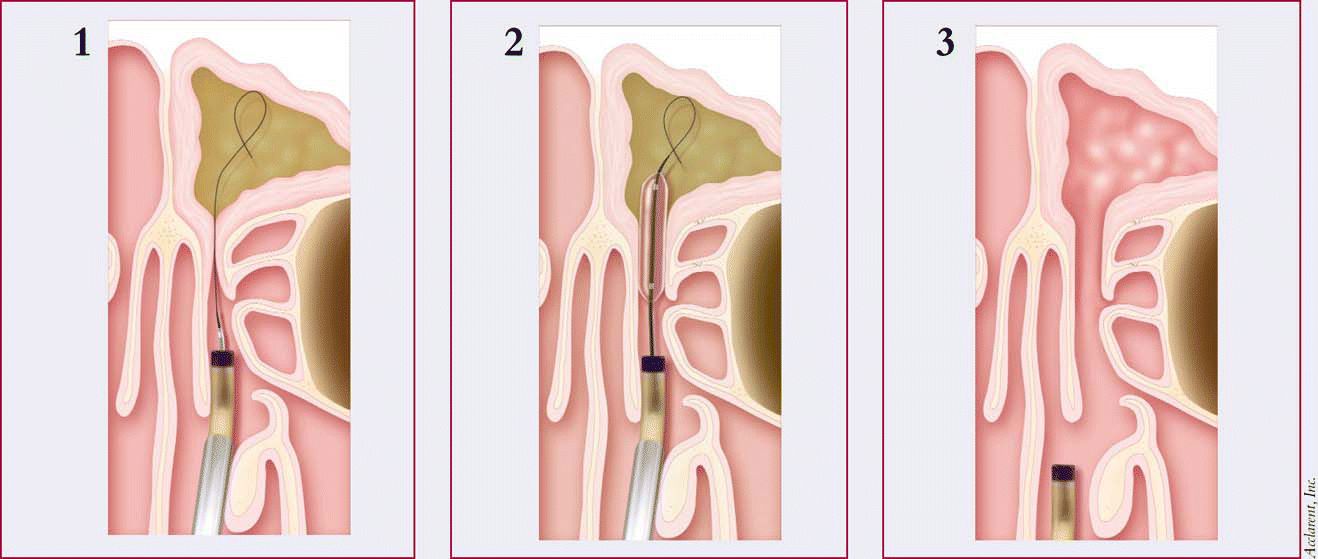
While the pilot trial was not designed to show efficacy, the device appeared to open blocked sinuses at least as well as standard endoscopic techniques, and patients reported improvement, he said.
Additionally, the studies suggested the device induces minimal trauma, Dr. Bolger noted. About two to three weeks after traditional endoscopic surgery, you need to debride the sinuses and remove the scabs and crusts inside. With the balloon, we observed we had fewer crusts and scabs to remove and less scar tissue to trim, which led us to speculate that there is less mucosal trauma, Dr. Bolger said.
For Now, Use Limited
The device is not for every patient with chronic sinusitis. At least for now, it is only indicated for use in the maxillary, sphenoid, or frontal sinuses-not the ethmoid sinus. In patients with blockages of the ethmoid sinus and at least one other sinus, we might perhaps use conventional endoscopy for the ethmoid and the new device for the other blocked sinuses. The two technologies can complement each other, Dr. Bolger said.
When the balloon expands, microfractures are created and are pushed out of the way, leaving the sinuses open and restoring normal sinus drainage and function. – -William E. Bolger, MD
Additionally, only otolaryngologists who have been trained by manufacturer can use the device. Any ENT can go through the training, Dr. Bolger said. But you have to go through the one-day training program in order to buy it.
The reason for this cautious approach: lack of long-term data when the device was approved in May 2005, Dr. Bolger said. The company wanted to take five or six months to identify and train eight to nine good surgeons in its use. As part of an ongoing clinical trial designed to obtain more safety and feasibility data and get more insight into outcomes, these surgeons have used the device to dilate over 300 obstructed sinus ostia in over 100 patients to date.
While six-month results of the trial will not be reported until this fall, early results suggest that outcomes hold up over time, said Dr. Goldberg, who was one of the first surgeons to be trained in the device’s use after FDA approval.
We are seeing patency in the vast majority of evaluated patients at two to three months, he told ENToday. This bodes well for the technology as you would typically expect closure of the ostium, if you were going to see it, at two to three months.
Dr. Bolger agreed. In general, when dilating ostia, you can usually tell in 6 to 12 weeks if it will work. Six months just gives it more acceptability.
Possible Drawbacks
There are some drawbacks. Dr. Goldberg said that one disadvantage of the technology is the need at this point to use fluoroscopy (for confirmation of guidewire and balloon placement) and its attendant equipment and radiation issues. However, the radiation exposure is quite brief and lessens with experience.
Donald Lanza, MD, Director of the Sinus & Nasal Institute of Florida in St. Petersburg, said that an even bigger drawback is that the new technology doesn’t target the underlying pathology. Dr. Lanza is not involved in the clinical trials of the balloon sinuplasty system.
Experimental studies show that in some patients with chronic sinusitis, the bone is chronically inflamed-even more so than in osteomyelitis, he said. This inflammation is thought to be caused by one of three insults: bacteria, fungus, or other microbes; enterotoxins (or superantigens) of bacteria such as Staphylococcus aureus; or biofilm, similar to the quiescent bacteria embedded in a polysaccharide matrix, which accumulates on one’s teeth overnight.
Some patients with chronic sinusitis have chronically inflamed bone, so that squashing the bone and stretching the tissue still leaves behind the underlying insult. You improve the plumbing temporarily but don’t necessarily remove pathology in surrounding tissue. – -Donald Lanza, MD
What this means, Dr. Lanza said, is that squashing the bone and stretching the tissue still leaves behind the underlying insult. You improve the plumbing temporarily but don’t necessarily remove pathology in surrounding tissue.
Conventional endoscopic surgery, on the other hand, removes the tissue-with the surrounding bacteria or other pathogen in it, he said.
First-Generation Device Intriguing
Nevertheless, Dr. Lanza said he was intrigued by the device and is scheduled to attend a training session on its use. The company has raised the possibility that future generations of the device could also contain a drug delivery system that could deliver antibiotics or other medications, he explained. That would be a real advantage.
The rationale for the device is that it gently dilates the sinus ostia to allow for better ventilation and drainage. – -Andrew N. Goldberg, MD
Until then, Dr. Lanza said he does not foresee the device having any effect on the treatment of chronic sinusitis. The technology will initially be embraced by ENTs, in part out of curiosity. But there are not enough data at this time to support its use over other measures.
Dr. Bolger agreed that it’s too early to change the standard of care for patients with chronic sinusitis. But, he added, it is only a first-generation device. In cardiology, they started with balloon angioplasty. Then restenosis problems led to angioplasty plus stenting and then to angioplasty plus drug-eluting stenting.
Who knows if a third-generation device, like drug-eluting stents in cardiology, will change the standard of care? he said.
©2006 The Triological Society



Leave a Reply