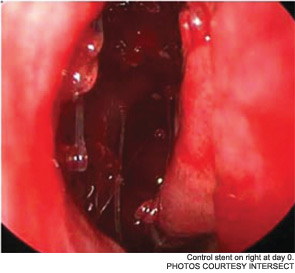
Maintaining sinus patency after functional endoscopic sinus surgery (FESS) for chronic rhinosinusitis (CRS) has long been an issue; as many as 23 to 47 percent of patients require revision surgery after FESS. Now, a new drug-eluting, bioabsorbable stent manufactured by Intersect (Palo Alto, Calif.) is being billed as a “breakthrough treatment [that] improves outcomes for sinus surgery,” according to a news release from the company. The device, which received pre-market approval from the U.S. Food and Drug Administration (FDA) in August, has been studied since 2008. It is currently available in Texas, New York, Philadelphia, New Jersey, Atlanta, Ohio and Kentucky.