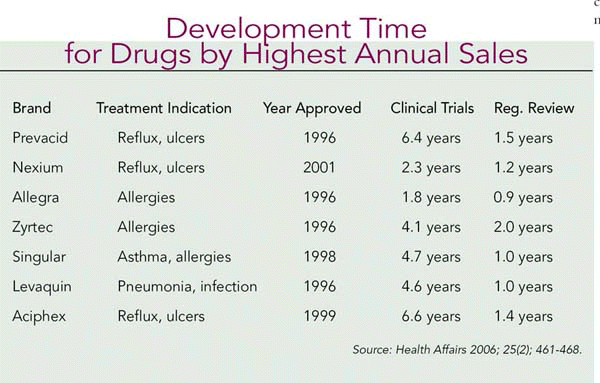
When comparing drug development times between 1985 and 1995, the average time was just over 10 years. For 1992 through 2002 the authors reported the median times for clinical trials and regulatory review were 5.1 years and 1.2 years, respectively. They conclude that the relative value of drugs in their therapeutic classes and market dynamics have more impact on cost than drug development times. Drugs with annual projected sales of $1 billion or more took one year less to develop than drugs with lower profit potential. (See Development Time for Drugs by Highest Annual Sales, above, for the clinical trial and regulatory times for some of the last decade’s blockbuster drugs.)
Explore This Issue
July 2006According to the authors, drug development costs averaged $868 million per NME, with a range of $500 million to over $2 billion, depending on therapeutic classes and the developing company.
Better Processes Can Encourage Innovations
Dr. Keyhani and colleagues also propose that improvements in the regulatory process, particularly FDA “fast tracking” of drug approvals, allow for speedier trials and lower clinical trial costs. The same legislation that allowed for fast tracking also permits faster development of orphan drugs.
The streamlined regulatory process has allowed Zila, Inc., a small pharmaceutical company based in Phoenix, to move swiftly with OraTest, an oral cancer-specific rinse being evaluated for its efficacy in early detection of oral cancers in high risk patients.
Zila received a special protocol arm from the FDA for Phase III clinical trials, which accelerates the trials and allowed Zila to cut its R&D costs by enrolling fewer than 4,000 high-risk patients in 13 sites including Johns Hopkins University, M.D. Anderson Cancer Center, and New York University’s College of Dentistry. Zila’s CEO Douglas Burkett, PhD, hopes that OraTest will improve oral cancer detection. “Currently, almost 70% of oral cancers are detected late in the disease, leading to low five-year survival rates. If the OraTest clinical trial succeeds, we hope to improve the survivability of this cancer,” said Dr. Burkett.
Still, the most recent research indicates that drug development times may not be a factor in rising drug prices, and that the costs of new drug development vary widely, from $500 million all the way up to $2 billion. While we may not like high prescription drug costs, Dr. Goldrich reminds us “high drug R&D costs are the price of progress.”
Leave a Reply