In 1982, the U.S. Food and Drug Administration (FDA) approved the first biologic, a recombinant insulin. Since then, the development and use of biologics has exploded. Between 2011 and 2016, the FDA approved 85 biologics (J Allergy Clin Immunol. 2017;139:1461-1464). Use of biologics—complex, protein-based medical treatments produced by living organisms—continues to grow yearly, and they are expected to eventually outpace traditional chemical drugs as medical treatments. But despite their promise, biologics come with one big drawback: high price tags.
Enter biosimilars, the “generic” version of biologics. Biosimilars are intended to be close-enough, even interchangeable, copies of biologic drugs. They’re relatively new here in the United States; the FDA approved the first biosimilar, filgrastim-sndz (Zarxio), which is clinically similar to Neupogen, in 2015. Since then, three more biosimilars have been approved: infliximab-dyyb (Inflectra), which is similar to Remicade; etanercept-szzs (Erelzi), which is similar to Enbrel; and adalimumab-atto (Amjevita), which is similar to Humira. These biosimilars are used to treat severe Crohn’s disease, arthritis, and psoriasis. More than 50 biosimilars are currently in the development pipeline, and biologics and biosimilars may soon become important therapeutic options in all areas of medicine, including otolaryngology.
Biosimilar Basics
© Peeradach R / shutterstock.com
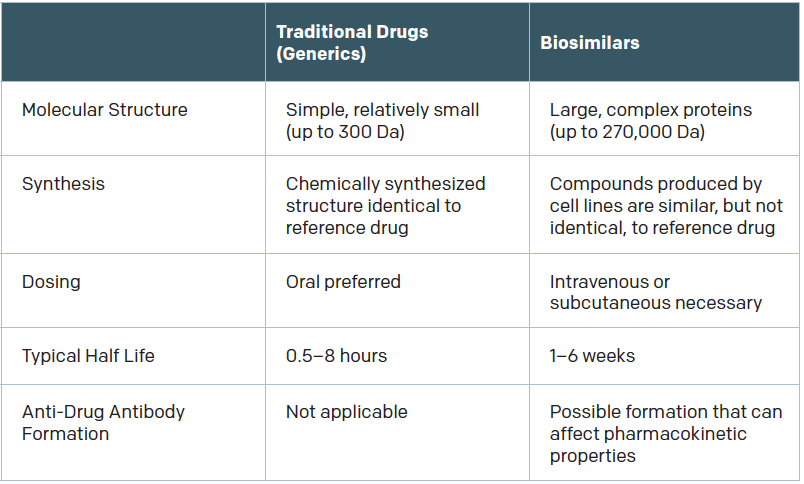
Biologics are made via complex processes involving living organisms. “Most of the biologics that are produced are from a single lab. They purify and harvest these antibodies from a mouse, chinchilla, or certain type of bacteria,” said Subinoy Das, MD, an otolaryngologist with Ohio ENT & Allergy Physicians in Columbus.
To make biosimilars, development labs “find the proteomic signature of all the proteins in the reference biologic and recreate that protein in the lab,” Dr. Das said. Recreating a complex biologic is not as simple as copying small-molecule drugs to make generics, however. “That’s simple chemistry,” Dr. Das said. “It’s much easier than trying to copy a complex monoclonal antibody that has protein folds and is very hard to replicate.”
As a result, biosimilars differ from their reference products. They have the same amino acid sequence, but “may differ in three-dimensional structure, glycosylation sites, isoform profiles, and protein aggregation,” according to Gary H. Lyman, MD, MPH, professor of medical oncology in the University of Washington’s School of Medicine in Seattle, who was quoted in a recent article for the American Society of Clinical Oncology (ASCO) (Published June 3, 2017). “Product drift because of minor differences in manufacturing, processing, and packaging may result in meaningful differences in both biosimilars and the originator biologic over time.”
The question, of course, is how significant are those differences? If the products are different enough, an immune reaction might be provoked. “There’s no question that there is some risk of immunogenicity when molecules aren’t 100% similar,” said Aaron Hakim, a third-year Yale medical student and co-author of a 2017 JAMA article on the adoption of biosimilars for chronic disease (JAMA. 2017;317:2163-2164). “If your immune system was fine with product A but product B is slightly different, it may cause adverse effects. The drug may no longer be effective for you.”
It’s important to note that immunogenicity is a risk even with biologics, because batch-to-batch variability exists in the production of therapeutic antibodies even when biologics are produced in the same plant, by the same manufacturer, and using the same process.
An International Perspective
Europe is far ahead of the United States in adopting biologics and biosimilars. In fact, the European Medicines Agency (EMA) approved 109 biologics between 2011 and 2016, compared with the 85 approved by the FDA in that same time frame (J Allergy Clin Immunol. 2017;139:1461-1464). Thirty biosimilars have already been approved in Europe, compared with just four here in the U.S.
What accounts for the difference?
“The regulatory paradigm for the approval of biosimilars in the United States really only started with the Affordable Care Act,” Hakim said. The Biologics Price Competition and Innovation Act passed in 2010 as part of the Affordable Care Act, and it is “only in the last several months that the FDA has put out guidance for what it required to be an interchangeable biosimilar, meaning a biosimilar that can be substituted for the branded biologic, at the level of the pharmacy,” Hakim said. That document was originally expected before the end of 2015; it was January 2017 when the FDA released draft guidance for industry regarding how to demonstrate interchangeability.
In contrast, the Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Quality Issues went into effect in 2006 in Europe. There, biosimilars must be proven similar to a reference biologic in terms of safety, efficacy, and quality, but drug manufacturers do not need to conduct clinical trials as extensive as those required for approval of the original biologic. (It is expected that FDA approval of biosimilars in the U.S. will require switching studies to demonstrate that patients can alternate between the original biologic and the biosimilar without diminished efficacy or serious side effects.)
Results from Europe indicate that biosimilars are generally very safe. “In Europe, patients are switching from a branded biologic directly to a biosimilar and maintaining the same level of disease control without developing safety issues,” Hakim said.
What Otolaryngologists Need to Know
Biologics are relatively new within the field of otolaryngology, and, so far, no biosimilars have been FDA approved for otolaryngologic indications. But given the emergence and promise of biologics in the treatment of allergy, asthma, and chronic rhinosinusitis, that may change.
Omalizumab, mepolizumab, and reslizumab are FDA approved for asthma, omalizumab is used to treat severe allergy-related asthma, and mepolizumab and reslizumab are approved for eosinophilic asthma. Benralizumab, an anti-IL-5 receptor alpha antibody, is currently being investigated as a treatment for severe asthma, and dupilumab, an interleukin-4 receptor alpha antagonist, is being evaluated for atopic dermatitis. Nemolizumab, an investigational humanized monoclonal antibody against interleukin-31, is also being investigated for resistant atopic dermatitis.
In the near future, biosimilar use in otolaryngology will likely occur primarily in the areas of allergy and immunology. In Europe, seven of the 31 products that received a positive opinion from the EMA Committee for Medicinal Products for Human Use have an indication for allergic and immune diseases (J Allergy Clin Immunol. 2017;139:1461-1464).
Biologics and biosimilars may also play a role in the treatment of chronic rhinosinusitis. “There are a fair number of biologics that are being tested for chronic rhinosinusitis,” said Stacey Gray, MD, assistant professor of otolaryngology at Harvard Medical School in Boston. “The idea is that, hopefully, this will be helpful for our patients with really recalcitrant chronic rhinosinusitis, but we’re not there yet.” Some small studies have shown potential benefit, but “a lot of the data has not actually been published yet,” Dr. Gray added. Larger, randomized-controlled trials will be necessary to determine the efficacy of biologic—and perhaps biosimilar—treatment for rhinosinusitis.
Interchangeability Is Important
For biosimilars to take off in the U.S., experts say it’s likely that drug manufacturers will have to demonstrate—and the FDA will have to confirm—interchangeability between biosimilars and their reference biologics. (To date, the biosimilars approved in the U.S. have been deemed “highly similar.”)
Interchangeability will give both consumers and clinicians the confidence that one product is as effective as the other, and that confidence is necessary to convince people to switch from expensive biologics to likely cheaper biosimilars. In fact, until patients and clinicians are willing to switch to biosimilars, cost savings might not materialize as hoped.
“One of the things that could affect the market—and this happened in Europe—is that they didn’t do much switching in the beginning,” said Joseph Fuhr, PhD, adjunct professor of pharmaceutical and healthcare business at University of the Sciences in Philadelphia. “What happened was, if a doctor had a new patient, they would put them on the biosimilar but not switch anybody away from the original product.”
Dr. Fuhr expects what happened in Europe to happen in the U.S.: Uptake of biosimilars will improve, and costs will go down, when interchangeability is demonstrated.
“When small-molecule generics came out, there was a big outcry and a lot of skepticism,” said Hakim. “What is now an accepted fact in the U.S. medical system is that small-molecule generics are just as good; there’s no reason to be skeptical of them.
“I think the same thing is going to be true for biosimilars. Hopefully, as regulatory bodies and clinicians gain a level of comfort on the basis of rigorous scientific and clinical data, we’ll be able to use biosimilars with the same degree of comfort as we use small-molecule generics.”
Jennifer Fink is a freelance medical writer based in Wisconsin.
Take-Home Points
- Biologics and biosimilars may soon become important therapeutic options in all areas of medicine, including otolaryngology.
- Minor differences in manufacturing, processing, and packaging may result in meaningful differences in both biosimilars and the originator biologics.
- Biologics are relatively new within the field of otolaryngology, and no biosimilars have been FDA approved for otolaryngologic indications.
- Biosimilar use in otolaryngology likely will occur in the treatment of allergy, immunology, and chronic rhinosinusitis.
Hospitalists as Test Subjects
According to a 2014 study by the RAND Corporation, biosimilars will “lead to a $44.2 billion reduction in direct spending on biologic drugs from 2014 to 2024” (2017. Rand.org). In Europe, biosimilars typically cost at least 25% less than reference biologics. Will the United States see similar savings?
Maybe. “The federal approval of the first biosimilar in the U.S. was supposed to foster the development of new products that offered big discounts on some of healthcare’s most expensive treatments,” said Dr. Das. “But there’s been no flood of new drugs and no lower prices since the FDA’s approval of Zarxio.”
One key to potential savings will be the FDA requirements for interchangeability. If the FDA requires extensive, expensive clinical testing to prove interchangeability, cost savings may not be as significant as anticipated.
The European experience, though, suggests a trend toward decreasing price as the biosimilars market matures. “As the market has evolved, we’ve seen discounts of 30 percent, even 75 percent,” said Dr Fuhr. Furthermore, in some cases, manufacturers of reference biologics are decreasing the cost of their drugs as well. As a result, he said, “access is increasing and costs are going down.”
He predicts that cost savings will appear here as well. “In five years, you should see considerable decreases in price.”