An FDA-cleared, noninvasive treatment approach that utilizes neural stimulation to desensitize patients to the disturbing impact of tinnitus has achieved consistently positive results in a controlled clinical study in Australia. In setting up this study we sought to set a much higher hurdle for a clinical success than others have done in the past, said Peter J. Hanley, PhD, CEO of the Australian entity Neuromonics.
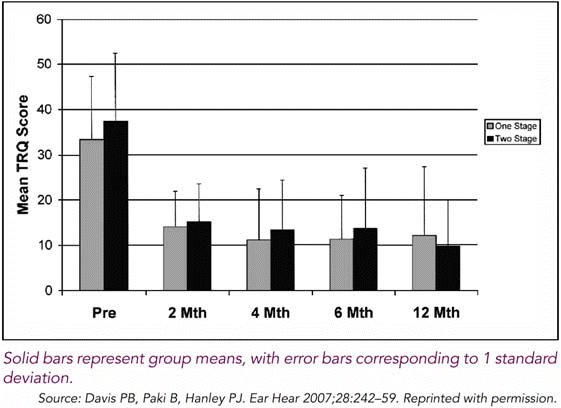
Two prior clinical trials for this approach have been conducted in the last decade. What was pleasing in the third trial was to see an even quicker benefit and even more significant outcomes, said Dr. Hanley. We set our benchmark for success at an improvement of at least 40 percent in tinnitus disturbance, and over 90 percent of the patients met or exceeded that hurdle after 6 months.1
The device and treatment, called the Neuromonics Tinnitus Treatment, combines the novel use of acoustic stimulation with a structured program of counseling. The distinctive acoustic component provides stimulation to auditory pathways deprived by hearing loss, engages positively with the limbic system, and allows intermittent, momentary tinnitus perception within a pleasant and relaxing listening experience, thereby facilitating desensitization to the tinnitus signal. By gradually breaking down the patient’s ability to detect the tinnitus, the amount of sound needed to cover up the tinnitus lessens over time. Patients become progressively less aware of and less disturbed by their tinnitus.
In the clinical trial, 35 subjects with predominantly moderate-to-severe tinnitus-related distress were randomly assigned to one of two treatment groups, corresponding to two stage-based variations of the treatment. In the first group, subjects were exposed to intermittent tinnitus perception throughout the six-month treatment program; in the second group, subjects’ tinnitus perception was completely covered up for the first two months and then only intermittently covered for the remaining four months of treatment.
After initial assessment and instructions, participants were given a personal sound player with earphones and an acoustic stimulus that was customized for their individual audiometric profile. Patients were instructed to use the instrument for at least two hours per day. At two, four, six, and 12 months after beginning treatment, both groups displayed clinically and statistically significant improvements in tinnitus disturbance, awareness, and minimum masking levels, as well as loudness discomfort levels. The Tinnitus Reaction Questionnaire (TRQ), comprised of 26 questions regarding the impact of tinnitus on the subject’s quality of life, was the primary measurement tool used in the trial (Figure 1).
In our study there was a quite a significant early benefit from the treatment, which happened in the first couple of months, said Dr. Hanley. Other treatment modalities that have been available for some time, which are heavily counseling-intensive, set the expectation of a year and a half to two years before the patient experiences significant benefit.
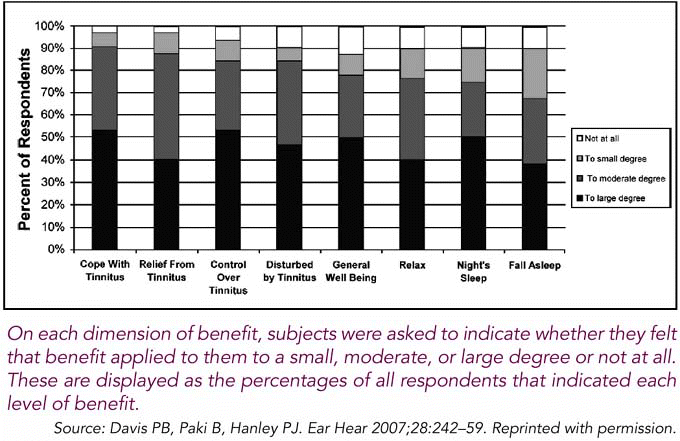
There were good rates of acceptability, convenience, and ease of use for the patients. More than 95 percent rated those questions positively in our treatment review survey, Dr. Hanley said. That has a major bearing on the relationship between the clinician and the patient. He added that this is also a time-efficient treatment that can be viably integrated into a physician’s practice (Figure 2).
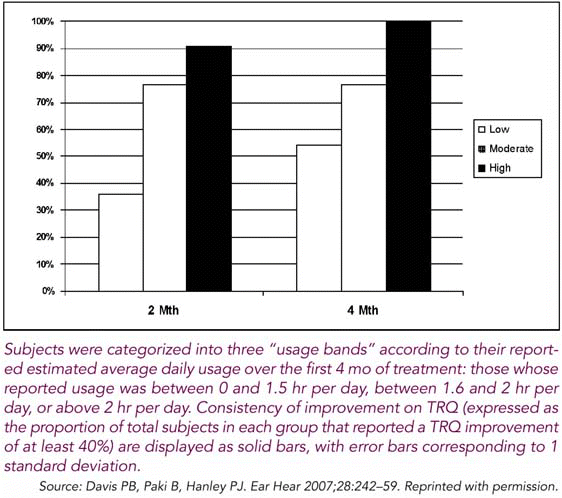
Clinical outcomes were found to be related to the level of usage of the acoustic stimulus. One of the most striking results in the third trial was the dosage effect that we found, said Dr. Hanley. There was a strong relationship between the hours of usage per day-that is, the amount of the therapeutic agent that the patients receive-and the speed and consistency with which the benefits were reported.
Furthermore, in the version of the technology that is being employed since the time of the third trial, a dosage meter can now be used to record patients’ usage. We are seeing highly compliant usage, and that’s another big point of distinction compared with [other] treatment options, he said.
Neuromonics in Practice
Jack A. Shohet, MD, otologist-neurotologist in the solo practice of Shohet Ear Associates in Newport Beach, CA, has been using the treatment with a number of his patients for the last year. His patients have had a variety of responses but they are mostly favorable. There are some patients who feel an instant relief the minute they put the device on their ears, he said, but that’s certainly the exception rather than the rule. Others experience that their tinnitus awareness and disturbance are slowly improving. And some patients don’t realize the improvement until we point out how they were at the very beginning of the treatment plan.
Dr. Shohet appreciates the approach’s well-defined treatment protocol that gives the patient control. We have a limited armamentarium for the treatment for tinnitus, he said. Many of these patients are looking for something they can do, and for a lot of them, having the feeling that they are doing something for their disease process, in and of itself is a benefit.
Dr. Shohet emphasizes that the first step in treating tinnitus is identifying the origin of the tinnitus and treating any medically or surgically treatable cause. After that, patients need to address lifestyle issues that affect their tinnitus. I call them the avoidance factors: avoid stress, fatigue, caffeine, and other loud noises, he said. And for anyone who has a hearing loss, we get them into a hearing instrument. If all those things fail, we typically will go to our Neuromonics [approach], and in our practice that is a pretty significant proportion [of patients]. Because of the fluctuating nature and intensity of their tinnitus, patients with Ménière’s disease and cochlear hydrops are not ordinarily good candidates for this treatment, although some inpatients in a nonacute phase of their symptomology have experienced some success. Other patients who would be considered poor candidates for the treatment are those who would have trouble self-administering it.
Any new treatment for tinnitus deserves some healthy skepticism, said Dr. Shohet. There have been a lot of things that have come up in the past that have been touted as the next greatest thing for tinnitus and in the end it hasn’t turned out to be much better than a placebo, he said. It is possible that with further studies we may find this instrument’s place in our armamentarium, but so far our early experience has been favorable.
Jack J. Wazen, MD, Director of Research at the Silverstein Institute in Sarasota, FL, helped lead the planning phase for the US trial, which is slated to enroll approximately 100 patients. Dr. Wazen, the first research fellow at the Silverstein Institute and now a partner in that practice, left Sarasota in 1983 and worked as a researcher, professor, and practitioner with Columbia University and the Columbia-Presbyterian Medical Center in New York. Because of my academic background and interest in research, Neuromonics approached me and asked if I wanted to participate in coordinating a national research protocol, he said. Approval of that protocol is imminent.
Because tinnitus is not one disorder, different people may react differently to various treatments. One of the fallacies is that there is going to be a cure for tinnitus, said Dr. Wazen. There may be a cure for a certain type of tinnitus that may not work for others. The advantage of the Neuromonics device is that it works on what all types of tinnitus have in common: the brain.
Because the treatment works to dissociate the sound the patient hears from the rest of emotional consciousness, he said, although the patient may still hear the sound, it is not depressing, it is not aggravating, it is not causing anxiety.
To Dr. Wazen, the principle of the Neuromonics treatment is attractive because dissociating the tinnitus from the rest of the patient’s brain activity would mean that patients can continue thinking, working, and enjoying their lives while their tinnitus is in their subconscious. That’s a big step in the right direction, he said (Figure 3).
Being cautious about what to tell patients is important to symptom management. What I tell my colleagues and particularly my patients is, ‘Although you may have heard and read that there is no cure for tinnitus, you should not assume every tinnitus is from the same reason.’ Dr. Wazen said. I want to make sure this tinnitus is not due to a tumor, like an acoustic neuroma, or other treatable conditions, he continued. Until a more definitive procedure or treatment is discovered, he added, we can now still tell our patients, ‘We have different treatment protocols you can consider-medications and instruments-which could reduce the impact of tinnitus and have you live with it in a better state of mind.’
Although so-called answers for treating tinnitus have come and gone, this is something that does make sense, said Dr. Wazen. He is hopeful that his investigative team will be able to duplicate the good results of the Australian studies. Then patients become advocates for this technology, he said, and the patient would be the best example to other patients.
Reference
- Davis PB, Paki B, Hanley PJ. Neuromonics Tinnitus Treatment: third clinical trial. Ear Hear 2007;28(2):242-59.
©2007 The Triological Society


Leave a Reply