Consider a kiss. The bestower must be able to breathe while pursing his or her lips, so “if there’s no air flow through your mouth or nose, you can’t give somebody a smooch,” said Peter Belafsky, MD, PhD, principal investigator of the laryngeal transplant project at the University of California, Davis, Medical School.
In other words, one of the most iconic ways for humans to connect with each other requires a functioning larynx. Intrepid clinicians and researchers are using regenerative medicine techniques to help people whose ability to speak, sing, hear—or yes, kiss—has been impaired.
Engineered Trachea
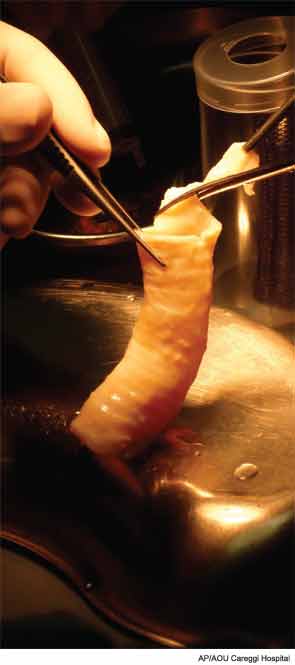
In Europe, an international team of surgeons and scientists has been transplanting stem cell-based tracheas since 2008. “We stripped the cells off the donor trachea, leaving just the extracellular matrix as a scaffold,” said Martin Birchall, MD, professor of laryngology at University College London Ear Institute. “Then we seeded the scaffold with stem cells taken from the patients’ own bone marrow.” The scaffold was bathed in a cocktail of hormones and growth factors to coax the growth of cell types associated with a normal trachea. In 2010, Dr. Birchall and colleagues, including pediatric cardiothoracic surgeon Martin Elliott, MD, performed the first pediatric, stem cell-based, tissue-engineered transplant, on a 10-year-old boy with congenital tracheal stenosis and pulmonary sling. He urgently needed a new trachea because the metal stent placed soon after his birth had eroded into his aorta, causing it to bleed. The patient, now 13, has a functioning airway.
But in early 2011, the team treated a 36-year-old man with a rapidly growing laryngeal tumor. This patient didn’t have the luxury of waiting for a donor trachea. Instead, bioengineers at the University College London developed an artificial laryngotracheal scaffold from plastic. Then, just as with a cadaver trachea, it was seeded with stem cells and incubated in the growth medium, and within two days, the patient had his new airway. A few months later, a 30-year-old man, also with an inoperable tracheal tumor, underwent a similar operation. These two patients became the first humans to receive stem cell-based organ transplants. So far, the original patient is doing well, but the second patient has died, for reasons seemingly unrelated to the implant. In the meantime, two more patients, both from Russia, have received engineered tracheas.
“There is a huge need to replace conventional organ donation, due to the infection risk and the risks associated with immunosuppression,” said Dr. Birchall. “We’d like to continue refining these techniques into good manufacturing processes. They are now quicker, cleaner and safer than ever before, and are ready for testing in clinical trials.”
Building a Better Larynx
The trachea is an attractive candidate for bioengineering, said head-and-neck surgeon Marshall Strome, MD, who is affiliated with New York’s Head and Neck Surgical Group and a group of collaborative airway specialists at Roosevelt Hospital that is studying tracheal transplantation. “Decellularized airway scaffolds, together with autologous or allogeneic hypo-immunologic stem cells, can be transplanted without the need for immunosuppression,” he said. He believes that synthetic tracheas hold great potential, but wondered, “What happens to those transplants over the long term?” He also pointed out that it is one thing to fashion a few inches of trachea but another to build a whole organ. “However, with increased understanding, we should be able to integrate genetically directed cellular products, and then, who knows?”
Still, the trachea is an easier target than the larynx, which Dr. Belafsky considers to be the most complex neuromuscular organ in the body. “We’re not there yet for a total laryngeal transplant, because you need sensation, among other things,” said Dr. Strome, who performed the first U.S. laryngeal transplant more than 14 years ago. Some investigators have proposed partial transplants using scaffolds made of cartilage and seeded with stem cells in much the same way as the tracheal implants were created. “This will permit the replacement of larger segments of the larynx than was previously thought possible, without the need for lifelong immunosuppression. But it will still require a functioning neuromuscular-cartilage complex, so you would have to leave the arytenoid cartilages behind.” Similarly, a tissue-engineered larynx currently would be suitable only for partial transplants, because “we need that functional myoneural-cartilage unit.”
Two years ago, surgeons at the University of California, Davis, led by D. Gregory Farwell, MD, performed the world’s second reported laryngeal transplant, on a patient of Dr. Belafsky. The larynx is unique, and uniquely difficult, for several reasons, Dr. Farwell said. First, unlike most transplanted organs, which perform single functions, the larynx has multiple complex jobs, providing not just an airway but also a vehicle for the voice and protection for safe swallowing. Also, unlike a liver transplant, a laryngeal transplant is not a life-saving surgery, although it can have a profound impact on the patient’s quality of life. “This intensifies the ethical considerations, with more attention given to specific risks and benefits,” he said. The surgery two years ago went well, but it was “humbling how challenging this was for us as surgeons,” Dr. Farwell added. Perhaps the most important lesson he learned was that “Dr. Strome’s initial laryngeal transplant success is reproducible. That has huge implications for the field.”
—Martin Birchall, MD, Univerisity College London Ear Insitute
Steven Zeitels, MD, professor of laryngeal surgery at Harvard Medical School in Boston, has been using cadaver aortas to reconstruct the larynx. “Patient 1,” on whom he operated in 2009 with thoracic surgeon John Wain, MD, was an 88-year-old man with laryngeal cancer who had failed radiotherapy but did not want a total laryngectomy. Dr. Zeitels credits Dr. Wain with the idea for the aortic patch. “You have an acellular piece of tissue that you’re putting into an environment with just the random blood supply from the muscles that are sutured into it,” he said. “The tissue is extremely easy to suture, like sewing a piece of rubber, and the aorta has a natural curve that can be fitted into the defect to form an adequate airway. And it is relatively sturdy; it does not fall apart.”
Success was far from guaranteed, Dr. Zeitels added. “Think of how hostile the environment is: You have an avascular piece of tissue and a random blood supply on only one side, and you’re not putting it in a part of the body where you can maintain sterility, [and] all kinds of flora exist in the human airway. There is also [gastric acid reflux] and, finally, the patient is coughing, so there’s barotrauma. On top of all of that, it’s an irradiated field. So it was stunning to me that it actually worked.” He estimates that approximately 25 patients have undergone the procedure so far, most of whom can start eating normally within a few days. Currently, the indications are for cancer and intractable stenosis.
As for “Patient 1,” he is now in his 90s, travels with his girlfriend and sends Dr. Zeitels a postcard every six months to let him know he is still doing well.
Building from Within
Tissue engineering is also being explored for its potential in the repair of craniofacial defects. At Washington University School of Medicine in St. Louis, head-and-neck surgeon Brian Nussenbaum, MD, is experimenting with bone morphogenetic proteins (BMPs) to regenerate bone in patients with defects related to trauma or oral cancer. Irradiated tissue is usually hypoxic or hypocellular, and the blood vessels may be scarred, which compounds the risks of reconstructive surgery, he said. BMPs, the central molecules involved in bone regenerative approaches, offer the potential for reconstructing tissue without many of the drawbacks associated with surgery. Essentially, “any bone regeneration strategy will involve BMPs,” he said.
Since 2004, Dr. Nussenbaum and his coworkers have used BMPs on six patients who required mandibular reconstruction due to damage from trauma or benign tumors. Of those six, five have had good results, but one patient experienced no bone growth. Cosmetic results in the five successful cases were good, with transient, superficial swelling as the only side effect.
The research is driven by its promise of real clinical benefit. “I’ve chosen to work in a stepwise manner, as this will have practical implications for the patients,” Dr. Nussenbaum said. He is trying to avoid one of the pitfalls of basic research, the “disconnect between ‘science’ and practicality.”
He also feels it necessary to proceed cautiously in what is still largely uncharted territory. “No one really knows yet how safe it is to use BMP therapies on someone who has had a mandible removed: Do these agents have an adverse effect on cancer cells? We still don’t know the answer.”
Gels with Cells
Tracheas are easier to reconstruct than larynges because of their function and composition, Dr. Nussenbaum said. “It’s easier to regenerate cartilage than it is to reconstruct the vocal folds. Also, the larynx is mobile, unlike the trachea. So the two organs have very different microenvironments.”
Nevertheless, several teams of investigators are studying ways to rebuild or repair the lamina propria in damaged vocal folds. “People have been treating the vocal folds with injections or moving the thyroid cartilage around for approximately 100 years,” said Dr. Zeitels. “The key unsolved problem is how you restore pliability to the vocal folds.” After working with singers, he said he’s witnessed how the loss of pliability affects the voice.
Much of the research in this field has focused on developing injectable hydrogels that serve as space fillers, exhibit mechanical properties similar to the natural tissue and can function in the unique environment of the vocal fold. Xianqiao Jia, PhD, associate professor of materials science and biomedical engineering at the University of Delaware in Newark, has been developing a hyaluronic acid (HA)-based complex gel network able to withstand vibratory stresses. “Whatever material you put in the vocal cords has to be able to handle a variety of sound amplitudes,” she said. These hydrogels also present minimal risk of rejection because they are non-immunogenic. Dr. Jia’s group has demonstrated the ability of HA-based microgels containing immobilized biomacromolecules for the slow release and prolonged presentation of various growth factors to induce tissue repair and regeneration. They hope to begin testing the gel in animal models within the next few months.
Dr. Jia is also collaborating with Susan Thibeault, PhD, of the University of Wisconsin in Madison, to develop a gel derived from resilin, a polypeptide isolated from insect wings. “It’s easy to mimic the viscoelastic properties of vocal folds,” said Dr. Thibeault. “We’ve been trying to load these gels with cells that can actually regenerate tissue.” Most of her recent research has focused on identifying the best type of cell to put in the gel, with emphasis on stem cells obtained from fat and bone marrow, and observing how those cells respond to vocal fold vibration.
Regenerating Hair Cells
Not all of the research on tissue regeneration has been centered on the aerodigestive tract. At Stanford University, Stefan Heller, PhD, in the department of otolaryngology, is leading efforts to coax mouse embryonic stem cells to differentiate into inner-ear cell types, such as hair cells. “We expose the stem cells to an environment that mimics embryonic development so we can watch them grow into these inner-ear cells. Basically, we are generating ears in a culture dish,” Dr. Heller said.
Those cells reliably turn into ear structures when placed in animal embryos; unfortunately, they are accompanied by other cell types that are tumorigenic. “That’s one of the problems we have to solve before we can start transplanting the cells into animal models.”
Ultimately, Dr. Heller foresees the development medication that can be placed in the ear to stimulate hair cell regeneration. “Our target is the supporting cells, which close the gaps left when hair cells die, and form an epithelium that is rather poorly characterized at the moment. So the goal is to find ways to stimulate these cells to divide and regenerate the lost hair cells. Birds and fish do it naturally, so it’s not so far-fetched.”
Admittedly, such products are far in the future, but Dr. Heller is optimistic. “We know what the roadblocks are,” he said. “We just have to shove them away.”
Leave a Reply