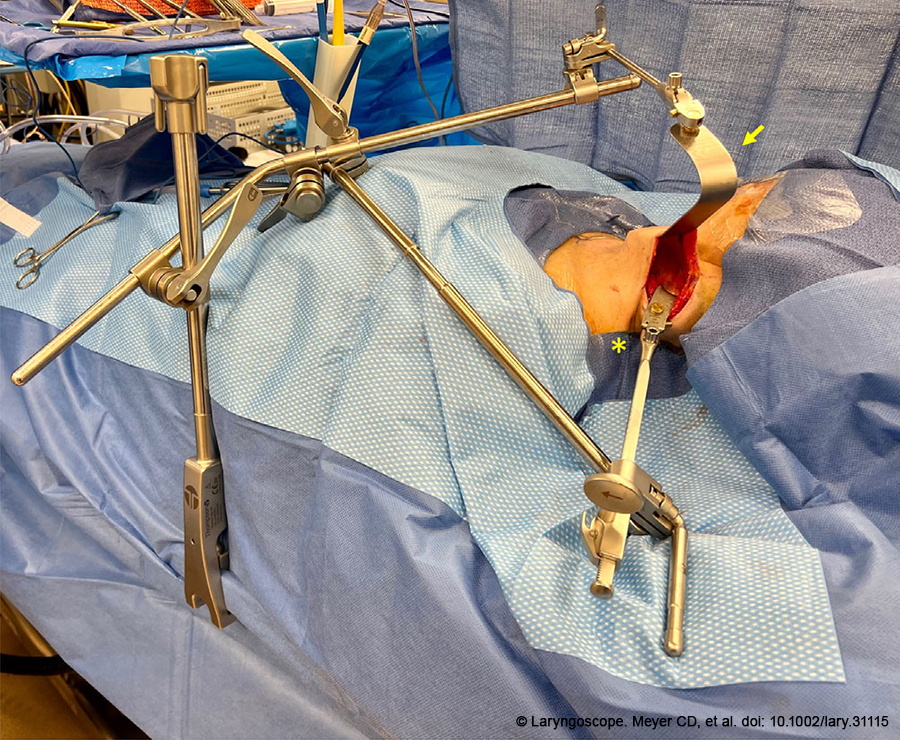
Figure 1. Positioning of the Thompson Retractor System prior to docking of the robotic system. The yellow arrow indicates the Deaver retractor attachment and the yellow asterisk indicates the concave lateral retractor attachment..
The patient was prepped, draped, and the incision line was injected with 2% lidocaine with 1:100,000 epinephrine. The skin was pulled anteriorly so the incision line sat on top of the posterior border of the SCM. This maneuver decreases the risk of proceeding below the SCM, which can place the spinal accessory nerve and branchial plexus at risk. The skin was incised sharply and a subdermal plane was elevated over the SCM until the lateral aspect of the platysma was identified, at which point dissection proceeded deep to the platysma. The external jugular vein and great auricular nerve were frequently encountered and were preserved. Subplatysmal dissection proceeded to the midline and until the thyroid cartilage and sternal notch were clearly visible. To retract the SCM posteriorly, the investing fascia was released medially, separating the anterior border of the SCM from the omohyoid and sternohyoid muscles. The Thompson Retractor System was then positioned (Figure 1) such that the skin envelope was elevated in the trajectory of the plane of dissection with the Deaver retractor attachment. The SCM was retracted posteriorly with the concave lateral retractor to reveal the carotid sheath. The NIM stimulating probe was used to stimulate the vagus nerve within the carotid sheath to verify proper functioning of the NIM system.
Explore This Issue
December 2023The Sp robotic system was positioned with the arms directed parallel to the plane of dissection. The camera was docked in the “up” position, with a monopolar cautery instrument superiorly, a Maryland bipolar forceps inferiorly, and a Cadiere forceps or a second Maryland bipolar forceps in the “down” position. For energy-based instrumentation, the ERBE generator was used and the senior author prefers effect settings of 5 and 3 for “Cut” and “Coag,” respectively. The omohyoid and sternohyoid were elevated off the thyroid to allow a broad plane of dissection, and then placed under the Deaver retractor attachment. The superior thyroid vessels were identified and ligated using the Maryland bipolar forceps. This allowed medial rotation of the thyroid and identification of the recurrent laryngeal nerve (RLN) within the tracheal esophageal groove. The NIM nerve stimulator was used to verify proper functioning. Dissection then continued inferiorly to identify and ligate the inferior thyroid vessels. Care was taken to identify and preserve the superior and inferior parathyroid glands with their associated blood supply by maintaining a capsular plane of dissection and minimizing monopolar electrocautery.
To complete the dissection of the RLN to the cricothyroid joint, the second grasping forceps in the “down” position was used to retract the thyroid gland ventromedially. This allows the inferior Maryland forceps to approach the RLN in the traditional inferior-to-superior fashion. Once the RLN was free of the thyroid gland and confirmed to be stimulating, the gland was again retracted dorsally and posteriorly by the inferior forceps, exposing the isthmus. The isthmus was then divided with bipolar or monopolar cautery. The specimen was removed, the wound bed was irrigated, and hemostasis was verified. The RLN and vagus were again stimulated to ensure proper function.
The skin was closed in layers with Monocryl sutures in a deep dermal fashion, with Dermabond skin glue superficially. A surgical drain was not necessary in our experience. The patient was observed in the postoperative recovery area for two to four hours and discharged home with scheduled follow-up in clinic in one to two weeks where repeat video laryngoscopy was performed to assess vocal cord function.
RESULTS
Ten patients underwent RT-PNA for thyroid pathology except for a single patient with a thymic cyst. Three had a prior history of keloids or hypertrophic scar. Mean operating time was 139 minutes, and there were no significant complications. There were no instances of temporary or permanent RLN injury. No patients required completion thyroidectomy.