Over the past decade, advances in digital imaging have transformed radiology departments by making it easier and faster for radiologic images to be shared among physicians via digital platforms, regardless of geographic location. Similar technology has advanced into the field of pathology, although the practice currently has limited approved application in the United States.
“Digital pathology is a generic term that refers to capturing microscopic images into a digital format,” said Thomas Bauer, MD, PhD, medical director for e-pathology in the department of pathology at the Cleveland Clinic in Ohio. Encompassed within digital pathology, he said, is the use of whole-slide imaging, which specifically refers to the use of high-resolution scanners to scan entire microscopic slides rather than isolated higher magnification fields.
Dr. Bauer and his colleagues have conducted a number of validation studies that successfully show that pathologists arrive at the same interpretation of an image whether it appears on a slide or is collected from a scan. These validation results were shown for routine pathology cases and difficult consultation cases, as well as when using frozen sections (Arch Pathol Lab Med. 2013;137:518-524; Arch Pathol Lab Med. 2014;138:1459-1465; J Pathol Inform. 2015;6:49).
Andrew B. Sholl, MD, director of autopsy pathology at Tulane University in New Orleans, called the benefits of digital pathology “quite extraordinary” and pointed out that the advantages of digital imaging over microscope slides include the ability of multiple pathologists to look at a case in real time and, at the same time, create a permanent digital record to allow for multiple reviews of the patient’s material. Additionally, he noted, the digitizing process allows for the ability to provide robust education for students and residents on difficult and unusual cases that may otherwise rarely be seen.
The drawbacks, he said, include the time it takes to digitize the images. “When scanning slides at 40x, it may take upward of 15 to 20 minutes per slide,” he said, which, he added, is not feasible for some cases, such as prostatectomy specimens that can range from 30 to more than 100 slides. “Another drawback is that the pathologist may feel uncomfortable that the provided image(s) do not portray enough data/histologic information to make a complete diagnosis,” he added.
Dr. Bauer also stressed that the time needed to interpret information from a scanned image is more than it would be from a microscope slide, particularly if used for primary diagnosis. “Pathologists have looked at microscope slides for a long time and we can do it relatively quickly,” he said. “The work stations we use to look at digital pathology images are not as fast as work stations in radiology, so the workflow of getting the patient information in front of us and the way you navigate between the images of each slide is not as efficient as it is for radiologists.”
I think the most useful application right now and in the near future is to allow us to provide subspecialty expertise quickly for consultation diagnoses to pathologists anywhere in the world. —Thomas Bauer, MD, PhD
Current Use of Digital Pathology in the U.S.
Other than for education and research, digital pathology in the United States as approved by the U.S. Food and Drug Administration (FDA) is currently limited primarily to consultation cases, providing a second opinion for diagnostic cases that are difficult or ambiguous. For Dr. Bauer, that is the best application of this technology. “I think the most useful application right now and in the near future is to allow us to provide subspecialty expertise quickly for consultation diagnoses to pathologists anywhere in the world,” he said, adding that he and his colleagues routinely provide consultations to labs in China, Hawaii, Abu Dhabi, and Florida using digital pathology imaging.
This use of digital pathology is particularly effective when consultations are needed in countries where there is a shortage of pathologists, such as China, according to Eric Glassy, MD, president-elect of the Digital Pathology Association and medical director of the Affiliated Pathologists Medical Group in Rancho Dominguez, Calif. For countries such as China, in which there is a shortage of pathologists as well as laws against sending tissue outside of the country, Dr. Glassy said that digital pathology provides a way to gain access to expert opinion outside of the country.
Dr. Glassy also emphasized that the shortage of pathologists in places such as China and Sweden has driven the interest and adoption of digital pathology for both primary and secondary diagnoses. Additionally, he stressed that the adoption of digital pathology in Europe and China has not been subjected to the constraints of the FDA, which is still reviewing the use of this technology in the U.S. for primary diagnosis.
Primary Diagnosis
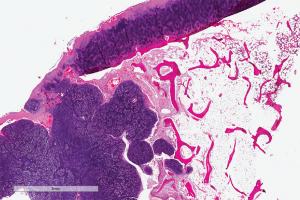
(click for larger image)
Digital slide depicting a whole-slide image of synovial chondromatosis, a lesion that sometimes involves the temporomandibular joint. Courtesy of the Center for ePathology, Cleveland Clinic.
At this time, the FDA has not cleared or approved any digital pathology whole-slide imaging device for use in making a primary diagnosis, according to an FDA spokesperson. Dr. Glassy elaborated on that point, stressing that the FDA regulation refers to the current regulation for device manufacturers and vendors that prohibits them from selling instruments labeled for primary diagnosis. “The FDA does not regulate the practice of medicine, so pathologists can use digital pathology for primary diagnosis,” he said, adding, however, that this use may involve medical-legal issues.
According to Dr. Glassy, interstate licensing issues only need to be addressed if digital pathology is used for primary diagnosis. Pathologists who make a primary diagnosis using a digital image sent from another state should have a license to practice pathology in the state from which the digital slide was delivered. Each state has different regulations, with 32 states currently allowing physicians to perform limited consultations across states and only a select number of states requiring state licensure.
The final technical guidance by the FDA on imaging and other issues (e.g., scan tolerances) was issued on April 20, 2016 in the report “Technical Performance Assessment of Digital Pathology Whole Slide Imaging Devices: Guidance for Industry and Food and Drug Administration Staff” (available at fda.gov).
Dr. Glassy and members of the Digital Pathology Association have participated in a number of meetings with the FDA to discuss the benefits of digital pathology and the potential risks that the technique may pose to patients. Dr. Glassy emphasized that, following these discussions, the FDA said that it would offer a less stringent review process for future digital pathology devices. Currently, digital pathology devices are categorized as class 3 devices, which are considered to carry the highest potential risk to patients and are therefore the most difficult to get approved. The FDA said that it would consider the next digital pathology device submitted for approval a “de novo” product, essentially making it easier for a new device to be approved.
Although FDA clearance is still pending for primary diagnosis, the FDA has cleared some digital pathology devices for image analysis) and for digital read of immunohistochemically-stained tissue slides, according to the FDA spokesperson.
According to Dr. Bauer, he and his colleagues are poised to adopt the use of digital pathology for primary diagnosis for a subset of cases once approved, given the successful results they’ve seen in their validation studies. “As work stations evolve and scanners become faster, there will be some role for digital pathology for primary diagnosis,” he said.
He expects that the first manufacturer to receive FDA approval for a digital pathology device to be used for primary diagnosis will probably occur later this year, with additional widespread approval and usage in the next year or two.
Mary Beth Nierengarten is a freelance medical writer based in Minnesota.
Key Points
- Whole-slide imaging refers to the use of high-resolution scanners to scan entire microscopic slides rather than isolated higher magnification fields.
- Digitized slides would allow multiple pathologists to look at a case in real time and create a permanent digital record to allow for future reviews of the patient’s material.
- The pathologist may also be concerned that the provided image portrays enough data/histologic information to make a complete diagnosis.
- Currently, the FDA has not approved any digital pathology whole-slide imaging device for primary diagnosis.
Additional Resources
- Clinical Guidelines for Telepathology
- Digital Pathology Association
- Sectra: Where and how digitization affects the pathology workflow
- Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center (Arch Pathol Lab Med 2013;137:1710-1722;)
Digital Pathology in Head and Neck Cases
For head and neck cases in particular, digital pathology can provide surgeons with rapid consultation diagnoses. “Many pathologists have a lot of difficulty with these tumors, so it is good for otolaryngologists and head and neck surgeons to recognize that this is a technology through which their pathologists can potentially seek quick consultations to get quick second opinions,” said Dr. Bauer.
However, he highlighted one of the limitations of digital pathology specific to head and neck cases: the need to provide surgeons with a quick assessment of resection margins using frozen sections. “We have learned so far in our validation studies that there are a few problematic areas that seem more difficult to interpret,” he said. “Perhaps we just need practice, but our experience so far suggests that squamous dysplasia may be more difficult to classify from digital images compared to microscope slides.”
“Often, and specifically when looking at margins, one wants to know the extent of dysplasia occurring on the mucosa, and that seems to be difficult to interpret digitally,” he said.—MBN
FDA Regulation
For digital pathology systems to be used in FDA-regulated laboratories, safeguards need to be met to comply with the following FDA stipulations:
Proper storage of digital slides, metadata, and information such as annotations and analysis results to ensure data integrity for the duration of image retention, which is two to five years.
Data management processes must be in place that include responsibility for and access rights to the archive.
Data security and protection of all data including any archived electronic records. This will require the existence of time-stamped audits of any activity that creates, modifies, or deletes data from the archive.
Source: Digital Pathology Association