This use of digital pathology is particularly effective when consultations are needed in countries where there is a shortage of pathologists, such as China, according to Eric Glassy, MD, president-elect of the Digital Pathology Association and medical director of the Affiliated Pathologists Medical Group in Rancho Dominguez, Calif. For countries such as China, in which there is a shortage of pathologists as well as laws against sending tissue outside of the country, Dr. Glassy said that digital pathology provides a way to gain access to expert opinion outside of the country.
Explore This Issue
August 2016Dr. Glassy also emphasized that the shortage of pathologists in places such as China and Sweden has driven the interest and adoption of digital pathology for both primary and secondary diagnoses. Additionally, he stressed that the adoption of digital pathology in Europe and China has not been subjected to the constraints of the FDA, which is still reviewing the use of this technology in the U.S. for primary diagnosis.
Primary Diagnosis
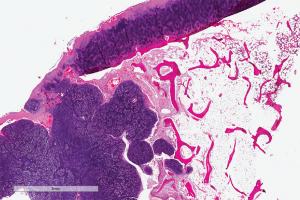
(click for larger image)
Digital slide depicting a whole-slide image of synovial chondromatosis, a lesion that sometimes involves the temporomandibular joint. Courtesy of the Center for ePathology, Cleveland Clinic.
At this time, the FDA has not cleared or approved any digital pathology whole-slide imaging device for use in making a primary diagnosis, according to an FDA spokesperson. Dr. Glassy elaborated on that point, stressing that the FDA regulation refers to the current regulation for device manufacturers and vendors that prohibits them from selling instruments labeled for primary diagnosis. “The FDA does not regulate the practice of medicine, so pathologists can use digital pathology for primary diagnosis,” he said, adding, however, that this use may involve medical-legal issues.
According to Dr. Glassy, interstate licensing issues only need to be addressed if digital pathology is used for primary diagnosis. Pathologists who make a primary diagnosis using a digital image sent from another state should have a license to practice pathology in the state from which the digital slide was delivered. Each state has different regulations, with 32 states currently allowing physicians to perform limited consultations across states and only a select number of states requiring state licensure.
The final technical guidance by the FDA on imaging and other issues (e.g., scan tolerances) was issued on April 20, 2016 in the report “Technical Performance Assessment of Digital Pathology Whole Slide Imaging Devices: Guidance for Industry and Food and Drug Administration Staff” (available at fda.gov).