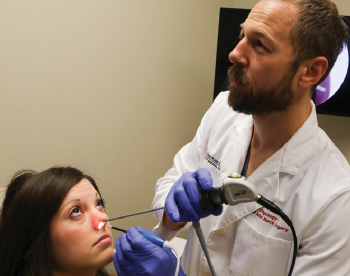
A physician at Ohio State University uses the Vivaer system.
© The Ohio State University/Wexner Medical Center
As technologies continue to evolve, so do the options otolaryngologists have for managing the myriad health concerns presented by their patients. Minimally invasive procedures are among the leading new technologies that are making it possible to do in the office setting what was once only done in the operating room.
Explore This Issue
September 2018Here are several novel technologies that are expanding the toolbox from which otolaryngologists draw to treat their patients.
New Procedure for Nasal Airway Obstruction
A new device that uses radiofrequency energy to modify the nasal valve area is showing promise as a novel way to treat symptoms of nasal obstruction. Called Vivaer (Aerin Medical, Sunnyvale, Calif.), the device is designed to remodel nasal tissues instead of ablating or removing the tissue, as previous energy-based technologies have been designed to do..
“Other recent technologies make use of an implant to support the nasal valve and reduce collapse, [which carries] a risk of implant extrusion,” said Michael J. Sillers, MD, an otolaryngologist at Alabama Nasal and Sinus Center in Birmingham. “Vivaer does not employ an implant.”
Instead, the device uses controlled energy that is applied intranasally to the internal nasal valve region to induce tissue shrinkage and tightening. This leads to “changing the shape and position of the caudal edge of the upper lateral cartilage without any incisions and within minutes,” said Dr. Sillers.
Results of a prospective, multi-center, non-randomized study of 50 patients treated with this device showed
substantial improvements in symptoms of nasal obstruction. Conducted by Aerin Medical, Inc., the manufacturer of the device, the study included patients treated by 16 physicians at eight study sites across the United States. All patients in the study had significant symptoms of nasal obstruction primarily caused by nasal valve dysfunction, with Nasal Obstruction Symptom Evaluation (NOSE) scores of higher than 60.
All patients were treated in a standard exam chair using the Vivaer device under direct visualization. Compared to baseline, the mean NOSE score improved by 54.8 points (69%) six months after treatment. Overall, 94% of patients had a 15-point or greater improvement in NOSE score following treatment. In addition, the ability to get enough air through their nose during exercise or exertion improved in 94% of patients, and 92% experienced improved sleep quality at night.
“As otolaryngologists, we were trained that the nasal valve is often the primary site of nasal obstruction; however, we have had few simple solutions to treat this area, so it has become largely overlooked,” said Dr. Sillers. “Vivaer is a simple and elegant solution that can be easily performed in the physician’s office. Once the diagnosis of nasal valve collapse is made, Vivaer offers a treatment to patients suffering from nasal obstruction beyond the traditional septum and turbinate surgeries.”
Dr. Sillers emphasized that using the device in an otolaryngology office is simple, requiring little equipment beyond the “small plug-and-play console and stylus,” and that several of his patients opted to undergo the Vivaer treatment during their initial consultation visit itself due to its simplicity and convenience.
As a new technology, the device’s one drawback is the lack of a reimbursement code; therefore, patients are required to pay for it out of pocket. Dr. Sillers suggested that patients are willing to pay for effective procedures such as this one, however.
“In our practice, like most everyone’s, we realized that patients increasingly pay large amounts out of pocket due to the rapidly changing insurance landscape,” he said. “Patients are more open to paying out of pocket for the benefits of an effective and convenient treatment.”
Cryotherapy for Excessive Rhinorrhea
For patients with excessive rhinorrhea, a novel device that uses cryotherapy to ablate the posterior nasal nerves (PNN) is showing beneficial results. Called Clarifix (Arrinex, Redwood City, Calif.), the device is the first of its kind to apply cryotherapy to the nose.
“This device is based on a well-known technology of cryoablation placed on a platform that allows for endoscopic visualization and specific placement where the posterior nasal nerves enter the nasal cavity,” said Amber Luong, MD, PhD, associate professor and director of research in the department of otorhinolaryngology–head and neck surgery and the Center of Immunology and Autoimmune Disease at the Institute of Molecular Medicine in Houston.
Results of the first series of patients treated with this approach showed that the device decreased rhinitis symptom scores for at least six months, and was safe and well-tolerated (Int Forum Allergy Rhinol. 2017;7:952–956).
Published in 2017, the study included 27 patients with chronic rhinorrhea and/or nasal congestion for more than three months. Patients were excluded from the study if they had self-reported history of chronic rhinosinusitis, severe septal deviation that prohibited visualization of the middle meatus, polyps or purulence in the middle meatus, septal perforation, or prior sinus or nasal surgery that significantly altered the posterior nasal cavity anatomy.
All patients underwent cryosurgical ablation of the PNN region. In the procedure, which was performed under local anesthesia, an otolaryngologist applied the cryotherapy device endoscopically to the posterior middle meatus to freeze the PNN region bilaterally.
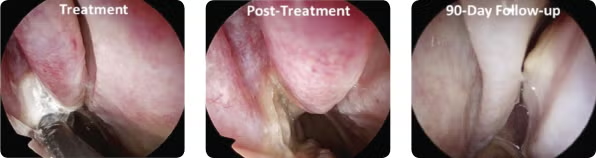
Endoscopic images from a patient at (A) treatment, (B) immediately posttreatment, and (C) 90-day follow-up.
© Arrinex, Inc.
Patients were then followed at seven, 30, and 90 days after the procedure and at each time period underwent a nasal endoscopy. In addition, they were interviewed at one, seven, 30, 90, 180, and 365 days to assess nasal symptoms and side effects.
The study used the Total Nasal Symptom Score (TNSS), a validated scoring system that rates severity of rhinorrhea, nasal congestion, nasal itching, and sneezing symptoms, as the primary outcome measure. All patients completed the TNSS prior to the procedure to establish a baseline score.
The study found a significant reduction in TNSS at 30 days after the procedure from baseline (2.6 vs. 6.2, respectively) in all 27 patients. This reduction continued to be significant at 90 days (2.7 vs. 6.2, respectively) in 24 patients, at 180 days (2.3 vs. 6.2, respectively) in 21 patients, and at 365 days (1.9 vs. 6.2, respectively) in 15 patients.
In addition to the reduction in symptoms, the procedure was safe and well tolerated, with 74% of patients reporting no or only mild pain/discomfort on the first day following the procedure. Other side effects experienced one day after the procedure, including severe ear blockage and severe nasal dryness, improved or fully resolved after 30 days. No patients reported dry eye.
Importantly, the procedure was done in an office setting. “This technology can be easily incorporated into the flow of an otolaryngology clinic,” said Dr. Luong. “It takes about 20 to 30 minutes to complete, with most of the time allotted to application of topical anesthesia. The treatment itself takes a little over a minute on each side.”
Like most new technologies, however, reimbursement remains a challenge, she said.
That said, Dr. Luong emphasized the initial promising results of the study and the benefits it shows in improving nasal congestion with minimal adverse effects. “This technology is important because post-nasal drainage is a very common complaint, and up until now the current treatment options require either a daily application or a procedure performed in the operating room under general anesthesia,” she said.
Balloon Dilation with Medical Therapy for Persistent ETD
Balloon dilation with medical therapy for persistent eustachian tube dysfunction (ETD) is a relatively new technology that has emerged over the past several years (Laryngoscope 2018;128:1200–1206; Otol Neurotol. 2018;39:894–902). Two systems are now approved by the Food and Drug Administration: the AERA Eustachian Tube Balloon Dilation System (Acclarent, Irvine, Calif.) and the XprESS ENT Dilation System (Entellus Medical).
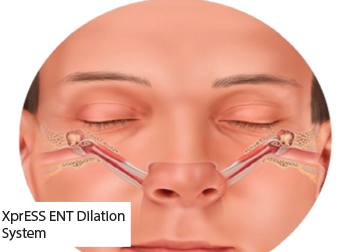
© Entellus
Recent studies by investigators using each of these systems demonstrated the superiority of this technology over medical therapy alone for patients with ETD (Laryngoscope 2018;128:1200–1206; Otol Neurotol. 2018;39:894–902). In May 2018, Poe and colleagues reported results of a multicenter, prospective, randomized, controlled trial that showed that patients treated with balloon dilation plus medical management had significantly greater tympanogram normalization at six weeks compared to those who received medical management alone (51.8% vs. 13.9%) (Laryngoscope. 2018;128:1200–1206). At 24 weeks, 62.2% of patients treated with balloon dilation plus medical management had tympanogram normalization. In addition, patients treated with balloon dilation and medical management reported significant improvement in symptoms based on Eustachian Tube Dysfunction Questionnaire-7 Symptom (ETDQ-7) scores at six-week follow-up compared to those treated with medical management alone (56.2% vs. 8.5%).
In June, Meyer and colleagues reported similar results, albeit based on the primary endpoint of ETDQ-7. In this study, patients treated with balloon dilation and medical management had a significantly mean change in overall ETDQ-7 score at six weeks of -2.9, compared with -0.6 for those treated with medical management alone. These improvements were maintained through 12 months for the patients treated with balloon dilation. The study also found that 66.7% of patients treated with balloon dilation and medical management had a significant improvement in tympanogram normalization at six weeks from baseline compared with 0% of patients treated with medical management alone.
Importantly, this last study also shows that this procedure can be done in the office setting. Unlike the study conducted by Poe and colleagues, in which all the procedures were conducted in the operating room, 72% of the procedures in the Meyer study were conducted in an office setting under local anesthesia.
According to lead author of the study, Ted A. Meyer, MD, PhD, director of the Cochlear Implant Center and associate professor in the department of otolaryngology–head and neck surgery at the Medical University of South Carolina in Charleston, patients generally tolerated the procedure as well in the office setting as in the operating room. As with any in office procedure, he said, pain management is a primary challenge.
An ongoing challenge with using this technology is reimbursement, he said. To date, there are no appropriate CPT codes for the procedure, so billing and insurance issues remain challenging. “There are lots of financial issues that still are needing to be worked out,” he said.
In addition, he emphasized that a main challenge is identifying the best candidates for the procedure. “We have a long way to go to know how many patients this will help and what criteria to use to pick the best patients,” he said.
Mary Beth Nierengarten is a freelance medical writer based in Minnesota.