The American Joint Committee on Cancer (AJCC) has announced a new edition of its staging manual for head and neck cancers. The revisions include sweeping changes in the classifications of some head and neck cancers and are scheduled to go into effect on January 1, 2018.
Explore This Issue
June 2017Dennis Kraus, MD, director of the Center for Head and Neck Oncology at the New York Head and Neck Institute in New York City, reviewed the update during a panel session at the Annual Meeting of the Triological Society. The changes are most dramatic in mucosal melanoma, oropharyngeal cancer, cancer with an unknown primary, and cancer of the oral cavity, he said.
The panel discussion also touched on robotic surgery and on the role of checkpoint inhibitors in head and neck cancer.
Changes to Cancer Staging
A main driver of the changes to the AJCC system was the goal to make staging a better tool for communicating with patients about their survival prospects, he said. “The concept is that we want to give any individual the actual likelihood of survival for their cancer,” he said. Those drafting the changes also wanted to refine the staging so that there would be a similar number of patients in each group, or roughly 25% in each of the four stages, he said.
Colored scanning electron micrograph (SEM) of squamous cell carcinoma cells from a human mouth.
© CNRI / Science Source
Another goal in making the changes is to distinguish each subgroup’s survival rates from those above and below it, he said. The changes also strive for worldwide acceptance and are meant to be applicable regardless of the resources of a center or provider, Dr. Kraus added.
Oropharyngeal cancer: Dr. Kraus said this is the category that has been updated the most. “One of the most notable changes—not just in the head and neck changes, but literally in this entire 600-page tome—are the changes that have been made in oropharyx cancer,” he said. Under the new system, only patients with metastatic disease will be considered stage IV. That’s because, when looking at three-year overall survival, stages I through III were grouped together fairly tightly, with “almost no statistical significance,” he said.
He added that, under the previous edition of the staging manual, the majority of patients were classified as having stage IV disease. “There’s poor balance, there’s poor hazard discrimination, and this was a must-fix for the current group,” he said. “We’ve made the obvious changes. We’ve separated oropharynx from hypopharynx. We have separate staging both for clinical and pathologic staging. And, interestingly, it’s very similar to nasopharynx disease, which has some overlap.”
There were no changes to the T (the size and location of the primary tumor) category, but many changes to the N (nodal) category. A clinical N1 designation will mean one or more ipsilateral lymph nodes, none greater than 6 cm; a clinical N2 will be contralateral or bilateral lymph nodes, none more than 6 cm; and clinical N3 will be lymph nodes larger than 6 cm.
Pathological N1 will be metastasis in four or fewer lymph nodes, and pathological N2 will be metastasis in more than four lymph nodes. There will be no pathological N3 category.
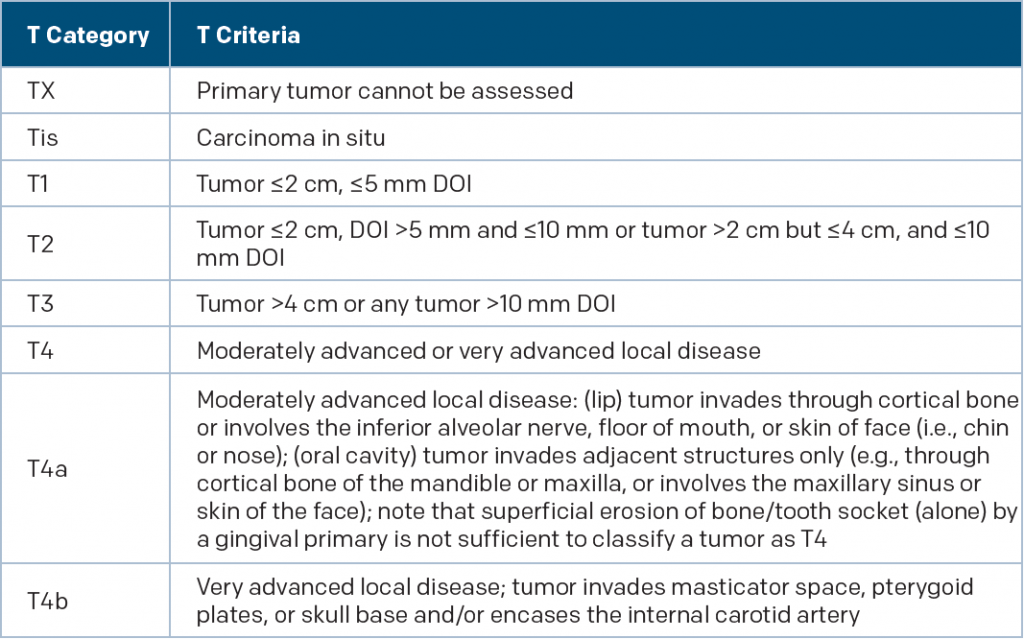
(click for larger image)
New T Category Criteria for Oral Cavity Cancer
DOI is depth of invasion and not tumor thickness
Source: AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017
Dr. Kraus acknowledged this might make for tricky conversations between patients and clinicians, who will have to “try to tell [patients] that what is stage IV disease as we sit here in 2017 really is stage I or stage II. And it does create some challenges.”
He added that smoking was not included in the staging system, despite the evidence suggesting its effect on risk because the data were just not strong enough yet to build it in. He anticipated it would be incorporated in the next edition.
Mucosal melanoma: Dr. Kraus said the main change here was an acknowledgement of the poor outcome that most patients tend to have with this disease. There will no longer be a T1 or T2 classification, only T3, T4a, and T4b, because the outcomes are just too dire, he said. Eliminating those levels is “one of the rare times this has ever occurred in the AJCC,” he added.
“Because of the universally poor outcome, I think this an appropriate amendment. Even when you are stage 3a, you have anticipated survival that’s approximately 40%, and as you get into the 4c you ultimately approach zero over time,” Dr. Kraus said. “In spite of our efforts, this population of patients continues to do poorly.”
Unknown primary that is p16-negative: Dr. Kraus pointed out that most unknown primaries end up being positive for p16 protein, associated with HPV positivity, but that this staging involves only the p16-negative variety. A notable change in this area is that the pathologic extranodal extension (ENE) is broken into two segments: “micro” ENE with extension of no more than 2 mm, and “major” ENE with extension of more than 2 mm. Pathological ENE will increase the N stage by one level from the previous system, he said.
Oral cavity cancer: The depth of invasion has been incorporated into the update, and will now increase the T category by one step for every 5 mm of invasion, Dr. Kraus said.
Dr. Kraus hopes the staging changes will improve care. “I think this will have a significant impact on our patients [and] a significant impact upon where we decide to both intensify and de-intensify patient care.”
Robotic Surgery
In another segment of the panel session, J. Scott Magnuson, MD, the chief medical officer of the Florida Hospital Nicholson Center in Celebration, Fla., said the onward march continues in robotic surgery, fueled by new technology geared toward augmenting the knowledge and skill of human experts; new financial models in which price is aligned with value and that promote technology that will lower the cost of treatment rather than increase it; and new roles for surgeons in which this technology could help boost the patient–provider ratio.
Dr. Magnuson said that most of the robotic surgery technology now in use is at Level 1, which allows the operator to keep continuous control of the system while the robot helps in some way. However, there are already robots made, although these are not yet commercially available, that are at a Level 3. At this level, the operator selects and approves a plan, and the robot performs the procedure automatically under close supervision. One such robot can suture after the surgeon sets it up, he added.
Another robot in the design phase is being devised to reach a Level 4—it is even able to make decisions, although it’s still under close supervision. “The ‘surgical cockpit’ is really the future for us,” Dr. Magnuson said. “If we’re to compare this to the airline industry and what pilots have— they’ve gone from manual control to computer controls to where the plane is flown automatically.”
Checkpoint Therapies
Ravi Uppalurri, MD, PhD, director of head and neck surgical oncology at the Dana Farber Cancer Institute in Boston, discussed the evolving role of checkpoint inhibition in the treatment of head and neck cancer, including the use of these therapies prior to surgery.
The therapies—which include nivolumab and pembrolizumab—have been shown to overcome the way in which a tumor can blunt the body’s immune response to cancer. They essentially “release the brakes” in the immune system’s fight against the tumor and have been shown to have lasting responses.
Some patients have had dramatic success with these types of therapy, with complete eradication of cancer in the neck, he said. “Ultimately, these kinds of vignettes really need to be confirmed with larger trials,” he added. “But I think these kinds of neoadjuvant approaches are really going to change how we manage our patients.”
Thomas R. Collins is a freelance medical writer based in Florida.
Take-Home Points
- A new staging system for head and neck cancers will go into effect in 2018 and will include sweeping changes in the classifications of mucosal melanoma, oropharyngeal cancer, cancer with an unknown primary, and cancer of the oral cavity.
- Robotic surgery continues to evolve.
- Some patients experience dramatic success with checkpoint inhibitor therapies, with complete eradication of cancer in the neck.
Further reading: Abstracts from The Laryngoscope
Recent Trends in Oropharyngeal Cancer Funding
Objectives/hypothesis: The incidence of oropharyngeal cancer (OPC) has increased in the United States. This has been driven by an increase in human papillomavirus (HPV)-positive OPC. Our objective is to determine trends in NIH-supported research funding and public interest in OPC.
Methods: The NIH Research Portfolio Online Reporting Tools database was evaluated for projects related to OPC between 2004 and 2015. Projects were evaluated for total funding, relation to HPV, principal investigator departmental affiliation and degree, and NIH agency or center responsible for grant. Google Trends was evaluated for relative Internet search popularity of oropharyngeal cancer and related search terms.
Results: In terms of NIH funding, 100 OPC-related projects representing 242 grant years and $108.5 million were funded between 2004 and 2015. Total NIH funding for OPC projects increased from $167,406 in 2004 to $16.2 million in 2015. Funding for HPV-related OPC increased from less than $2 million yearly between 2004 and 2010 up to $12.7 million in 2015. Principal investigators related to radiation oncology ($41.8 million) and with doctor of medicine degrees ($52.8 million) received the largest share of total funding. Relative Internet search popularity for oropharyngeal cancer has increased from 2004 to 2015 compared to control cancer search terms.
Conclusion: Increased public interest and NIH funding has paralleled the rising incidence of OPC. NIH funding has been driven by projects related to the role of HPV in OPC (Laryngoscope. 2017;127:1345–1350).
Impact of TORS on Overall Treatment of patients with Oropharyngeal Cancer
Objectives/hypothesis: To assess adjuvant therapy in patients undergoing surgical management of oropharyngeal squamous cell carcinoma (OPSCCA) with transoral robotic surgery (TORS) and neck dissection.
Methods: Patients undergoing treatment for OPSCCA were selected from a prospective protocol evaluating functional and oncologic outcomes following TORS with a comparator group of OPSCCA patients receiving definitive chemoradiotherapy (CRT) participating in a separate prospective protocol.
Results: Forty-two patients represented the TORS group and 38 the CRT group. Twenty (48%) of the TORS patients received surgery only, whereas nine (21%) underwent adjuvant radiotherapy and 13 (31%) adjuvant CRT. Adjuvant therapy patients had a higher overall T and N stage than the TORS-only group. Surgery resulted in stage changes in 18 (43%) patients, leading to alteration in therapy for nine (21%) patients. The 3-year overall survival (OS), disease-specific survival (DSS), and locoregional control was 74% versus 90%, 94% versus 94%, and 72% versus 91% for the TORS-alone versus TORS plus adjuvant therapy groups, respectively. Comparison with the CRT group revealed a survival benefit in the TORS group approaching significance, with a 3-year OS of 83% versus 57% and DSS of 94% versus 85%, respectively.
Conclusion: Primary surgical management of OPSCCA with TORS and neck dissection provides accurate staging information, which can lead to the appropriate selection of subsequent therapy (Laryngoscope. 2015;125 Suppl 10:S1–S15).
TORS for Oropharyngeal and Tongue Cancer in the U.S.
Objectives/hypothesis: To compare the clinical and cost outcomes of transoral robotic surgery (TORS) versus open procedures following the U.S. Food and Drug Administration approval in December 2009.
Methods: Elective partial pharyngectomies and partial glossectomies for neoplasm were identified by ICD, 9th Revision, Clinical Modification code.
Results: TORS represented 2.1% in 2010 and 2.2% in 2011 of all transoral ablative procedures. Patients undergoing open partial pharyngectomy for oropharyngeal neoplasms (n = 1426) had more severe illness compared to TORS (n = 641). However, after controlling for minor-to-moderate severity of illness, open partial pharyngectomy was associated with longer hospital stay (5.2 vs. 3.7 days, P < 0.001), higher charge ($98,228 vs. $67,317, P < 0.001), higher cost ($29,365 vs. $20,706, P < 0.001), higher rates of tracheostomy and gastrostomy tube placement, and more wound and bleeding complications. TORS was associated with a higher rate of dysphagia (19.5% vs. 8.0%, P < 0.001). The lower cost of TORS remained significant in the major-to-extreme severity of illness group but was associated with higher complication rates when compared to open cases of the same severity of illness. A similar analysis of TORS partial glossectomy for base of tongue tumors had similar cost and length of stay benefits, whereas TORS partial glossectomy for anterior tongue tumors revealed longer hospital stays and no benefit in charge or cost compared to open.
Conclusion: Early data demonstrate a clinical and cost benefit in TORS partial pharyngectomy and partial glossectomy for the base of tongue but no benefit in partial glossectomy of the anterior tongue. It is likely that anatomic accessibility and extent of surgery factor into the effectiveness of TORS (Laryngoscope. 2015;125:140–145).
