
© James Steidl / shutterstock.com
The 21st Century Cures Act, also known as the Cures Act, provides the National Institutes of Health (NIH) with critical tools and resources to advance biomedical research across the spectrum, from foundational basic research studies to advanced clinical trials of promising new therapies. Both the U.S. House of Representatives and the Senate passed it with strong bipartisan support, and it became law on December 13, 2016.
Explore This Issue
August 2017The legislation provides $4.8 billion in funding to four highly innovative scientific initiatives over the next 10 years, including the All of Us Research Program, formerly known as the PMI Cohort Program ($1.45 billion), the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative ($1.5 billion), Cancer Moonshot ($1.8 billion), and the Regenerative Medicine Innovation Project ($30 million) (See Table 1).
“Funding to the NIH has been one of the most critical drivers of improved health in the world,” said Joseph E. Kerschner, MD, professor of otolaryngology, microbiology, and immunology, dean of the School of Medicine, and executive vice president of the Medical College of Wisconsin in Milwaukee. “Virtually every disorder that an otolaryngologist comes into contact with has been altered, with improved patient outcomes, because of NIH-funded research. Specifically, the Cures Act includes increased funding to the NIH for cancer research—including cancer of the head and neck—which has the tremendous potential to make significant progress toward improved care and survival rates. Other areas of emphasis include funding for research in opioid use, behavioral health, and access to healthcare, which are critical areas impacting otolaryngologists and their patients.”
Baldwin Wong, chief of the Science Policy and Planning Branch of the National Institute on Deafness and Other Communication Disorders (NIDCD) in Bethesda, Md., believes the act supports the NIDCD’s mission and will benefit the field of otolaryngology in many ways. The NIDCD is one of 27 NIH institutes and centers within the U.S. Department of Health and Human Services.
Here’s a closer look at each of the four initiatives.
Funding to the NIH has been one of the most critical drivers of improved health in the world. Virtually every disorder that an otolaryngologist comes into contact with has been altered, with improved patient outcomes because of NIH-funded research. —Joseph E. Kerschner, MD
All of Us Research Program
For this program, NIH will recruit one million or more adult volunteers in the United States to participate in a study to accelerate research for a wide range of diseases and improve the understanding of health. “Participants will provide information about their medical history and lifestyles on a questionnaire and may also be asked to provide physical measurements or donate a blood or urine sample,” Wong said. “Individuals with communication disorders could be part of this volunteer group, as the study aims to reach a cross-section of the country.”
A database will house and provide secure access to the data. Private and public partnerships will be established among scientists and physicians to share the data and study genetic factors, environmental exposures, and lifestyles and their impact on diseases and disorders. “Otolaryngologists partnering in the study will have access to this data and might be able to identify susceptibility genes or environmental factors that contribute to sensorineural and hereditary hearing loss,” Wong said.
BRAIN Initiative
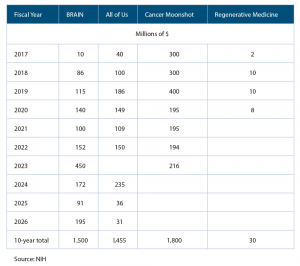
(click for larger image) Table 1. Funding for NIH Innovative Research Initiatives under the Cures Act
This facet of the Cures Act is aimed at revolutionizing our understanding of the human brain. Researchers intend to use new imaging tools to produce a revolutionary, dynamic picture of the brain that shows how individual cells and complex neural circuits interact in real time. “Researchers will be able to see which areas of the brain are involved in normal or disease processes,” Wong said. “Being able to visualize the affected areas of the brain in an individual with tinnitus using functional magnetic resonance imaging or having the ability to image auditory processing disorders or hyperacusis in the brain in real time would have great benefits.”
This imaging data could fill significant gaps in current knowledge and provide unprecedented opportunities for exploring how the brain enables the human body to record, process, utilize, store, and retrieve vast quantities of information, all at the speed of thought. Ultimately, researchers hope they will be better equipped to treat, cure, and even prevent brain disorders.
Cancer Moonshot
The Cancer Moonshot aims to accelerate cancer research to make more therapies available to more patients, while also improving the ability to prevent cancer and detect it early. Presently, the National Cancer Institute is asking scientists to submit grant applications for cancer research. “Scientists interested in studying head and neck cancers may submit applications for support as part of this initiative,” Wong said.
Regenerative Medicine Innovation Project
Regenerative medicine is an emerging area of science that holds great promise for treating and possibly curing a variety of conditions. The initiative promotes the use of adult stem cells and other technologies—such as engineered biomaterials and gene editing—to repair or replace damaged cells, tissues, or organs.
“If scientists could coax adult stem cells to regenerate new cells to replace lost or damaged hair cells in the inner ear after hearing loss, there may be a good chance that hearing could be restored,” Wong said. “Or, if researchers could repair damaged vocal folds, that may help alleviate some voice disorders.”
Looking Ahead
Wong is optimistic about the benefits that the Cures Act’s initiatives will bring. “Although a specific study may not find a cure, it could uncover new knowledge about normal and disease processes that could lead to a cure,” he said. “Or, it could provide the necessary basic research that eventually leads to a new treatment, such as a new assistive device or a new drug.”
Karen Appold is a freelance medical writer based in New Jersey.
Key Points
- The Cures Act provides the NIH with $4.8 billion in funding to advance biomedical research.
- Programs funded under the act focus on cancer research, regenerative medicine, opioid use, behavioral health, and access to healthcare.
- Some physicians are concerned that provisions in the act could actually harm the drug and device marketplace by encouraging the FDA to emphasize speed over science.
What to Look for in a Disability Policy
Another component of the 21st Century Cures Act is to build on the U.S. Food and Drug Administration’s (FDA) ongoing efforts to advance medical product innovation and ensure that patients get access to treatments as quickly as possible, with continued assurance from high-quality evidence that they are safe and effective, stated Robert M. Califf, MD, commissioner of the FDA.
“The FDA’s approval process for pharmacologic agents and devices is designed to protect patients and ensure quality and safety,” said Joseph E. Kerschner, MD, dean of the School of Medicine and executive vice president of Medical College of Wisconsin in Milwaukee, and chair-elect of the Council of Deans for the Association of American Medical Colleges, based in Washington, D.C. “However, the process is expensive and time consuming, which can delay the ability for patients to access cutting-edge therapies. The Cures Act admirably attempts to continue to safeguard FDA approvals appropriately, but will also look at ways that enhance efficacy and efficiency in bringing new therapies to market without lessening patient safety. The act may provide some beneficial changes to regulations, which will help streamline processes and deliver new technologies more effectively.”
But Michael S. Sinha, MD, JD, MPH, a postdoctoral fellow in the Program On Regulation, Therapeutics, And Law (PORTAL) at Boston’s Brigham and Women’s Hospital/Harvard Medical School, and some of his colleagues are concerned that some of the act’s provisions could actually harm the drug and device marketplace by encouraging the FDA to emphasize speed over science and rely on less rigorous data for approving new products and indications.
While the FDA was slow to give approvals 30 years ago, that is not true today, Dr. Sinha said. Currently, the FDA is among the fastest drug regulatory agencies in the world. A Yale study showed that between 2011 and 2015, FDA approvals were, on average, 60 days shorter than approvals from the European Medicines Agency (N Engl J Med. 2017; 376:1386-1387). This is consistent with results from a previous study by the same author group that looked at approvals from 2001 to 2010. One reason for the increase in speed is that the FDA has several expedited development and approval pathways, and approximately three-quarters of the drugs approved in 2016 qualified for one or more of these pathways. In addition, 95% of all new drug approvals are now approved on the first cycle of review. Increased review speed has been associated with problematic safety outcomes, however. One study published in JAMA found an increase in post-approval safety events from 2001 to 2010, with higher events noted among accelerated approvals and near-deadline decisions (JAMA. 2017;317:1854-1863).
Dr. Sinha, along with other members of the PORTAL Research Group, noted in Health Affairs Blog and JAMA that they are concerned about the impact of the Cures Act on patients (JAMA. 2017;317:581-582). “Allowing drug approvals on the basis of less rigorous data—such as through the limited population pathway for antibiotics—will lead to numerous products becoming available without clear evidence of benefit to guide physician and patient decision-making,” he said. For example, it will be increasingly difficult for physicians to base their confidence in a drug’s safety and efficacy on FDA approval alone, particularly as biomarkers and other surrogate measures replace meaningful clinical endpoints in pivotal studies leading to approval. “Of course, all of these products will invariably be extremely expensive as well, which will put a strain on limited healthcare budgets and possibly detract from already available, well-proven therapies.”