INTRODUCTION
Thyroidectomy using transaxillary robotic surgery (TARS) was first developed in 2007 using the da Vinci robot. It is used as a safe and efficient alternative to conventional cervicotomy to remove anything from small thyroid nodules to Graves’ disease goiters and cervical lymph nodes in different populations (World J Surg. 2018;42:393-401; Laryngoscope. 2011;121:521-526). Advantages of the technique are cosmetic, a high-definition view of structures, and a reduced risk of compressive cervical hematoma (due to a large surgical space); the main drawbacks are the cost and duration of the procedure (World J Surg. 2018;42:393-401; Laryngoscope. 2011;121:521-526; Ann Surg Oncol. 2011;18:226-232; Head Neck. 2015;37:1705-1711). Another important hurdle is the learning curve (Ann Surg Oncol. 2011;18:226-232; Head Neck. 2015;37:1705- 1711), as robotic surgery requires a new set of skills; training is required before the first operation can be performed. Furthermore, the transaxillary approach has its own learning curve, as it is uncommon for head and neck surgeons to laterally approach the neck midline.
Explore This Issue
November 2021In this article, we wish to help reduce the learning curve of the transaxillary approach for robotic thyroidectomy by providing a step-by- step description of the procedure, highlighting tips and pitfalls.
METHOD
All consecutive transaxillary approaches for robotic thyroidectomy between 2010 and 2018, performed by the same head and neck surgeon, were retrospectively analyzed (duration and complications), for the analysis of the learning curve. All patients had been given a choice between conventional cervicotomy and the transaxillary robotic approach. Procedures took place in either an adult or a pediatric tertiary center, and a da Vinci robot was used for the subsequent thyroidectomy.
Surgical Technique
Preparation and positioning. Under general anesthesia, the patient’s axilla is exposed by fixing the arm over the head with a 90° to 100° flexion of the elbow. The arm should rest in a natural fashion, over the forehead, so as to limit the risk of brachial plexus injury. The head is slightly turned toward the contralateral side of the incision and neck extended. Anatomical landmarks are drawn on the patient’s skin and may include the sternal notch, cricoid and thyroid cartilage, mandible angle, and sternocleidomastoid (SCM) muscle. The skin incision 1 cm posterior to the anterior axillary fold and the surgical corridor are also drawn. The arm can be moved back to its natural position to check that the scar will be well hidden. These landmarks must remain visible when draping the patient to be able to check for skin integrity during the procedure and allow conversion to cervicotomy if necessary.
The assistant is on the opposite side of the patient to hold the retractors without hindering the surgeon. Farabeuf and vaginal- valve retractors are required and should include a canal for smoke suction. Long instruments (30 cm) are necessary to reach the neck midline from the axillary incision.
The procedure is best performed using a headlamp and surgical loupes, especially to aid precise hemostasis of perforating arteries during muscle dissection. The da Vinci robot endoscope may also be used to increase visibility and for teaching (see supporting video) but requires a second assistant to manipulate it. The view from the endoscope may also help the assistant holding the retractors to obtain the best exposure possible by giving direct feedback.
Incision and approach to neck midline. A 4- to 6-cm skin incision is performed immediately posterior to the anterior axillary fold, following the axillary hairline (thus the scar is hidden in the axilla), and the lateral border of the pectoralis major is exposed. Using monopolar electrocautery, dissection is performed above the pectoral fascia toward the clavicle and the SCM. A thin layer of fat tissue may be left on the pectoral muscle fascia so as not to expose perforating arteries, which can retract into the muscle and cause delayed bleeding.
Before opening the SCM muscle, the surgical corridor is widened superiorly and inferiorly to maximize exposure and visibility. The dissection should remain in a subplatysmal plane above the clavicle by following the lateral border of the clavicular head of the SCM. Thus, originating from the SCM, the dissection should proceed laterally until two fingers can comfortably be inserted in the workspace. Great care must be taken, as the skin and muscle covering the clavicle are very thin and the external jugular vein is superficial immediately beneath the platysma.
Neck midline and exposure of thyroid gland. Once the sternal and clavicular heads of the SCM have been identified, the neck midline is approached by dissecting between both branches. Electrocautery dissection should remain close to the sternal head of the SCM to prevent any injury of the internal jugular vein (IJV), which lies at the same depth and is immediately medial to the clavicular head.
The sternal head is elevated, exposing the strap muscles. The omohyoid muscle is an important landmark that must be identified, as it is immediately superior to the IJV and usually indicates the upper pole of the thyroid gland. As with the SCM, dissection must remain close to the omohyoid to prevent IJV injury. The omohyoid muscle can be sectioned if a larger workspace is required (lymph node dissection or large gland).
The strap muscles are elevated as the dissection progresses, exposing the thyroid gland. Again, dissection must be careful, and the monopolar electrocautery should remain in direct contact with the inferior border of the strap muscles to keep a safe distance from IJV. In case of IJV injury, direct ligature or vascular clips (3 or 5 mm) may be used.
Dissection must be pursued until the contralateral omohyoid muscle is reached (Figure 1). Thus, the da Vinci Kuppersmith retractor can be put into place, elevating the skin and creating a workspace sufficient for robotic dissection of both thyroid lobes.
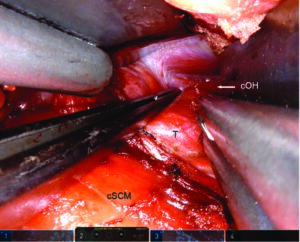
Figure 1. Final view with thyroid and landmarks. Right transaxillary approach. The forceps retracts the thyroid gland downward
to expose the contralateral omohyoid muscle, which is the last landmark to be seen before the final Kuppersmith
retractor is put in place. cOH = contralateral omohyoid muscle; cSCM = clavicular head of the sternocleidomastoid muscle;
T = thyroid gland. SIMON F, ET. AL. LARYNGOSCOPE. DOI:10.1002/LARY.29753
Closure After Robotic Surgery
It is of paramount importance to check the entire workspace for bleeding, especially from the muscle tissue, to prevent hematoma. This is especially true during the first cases of TARS for inexperienced surgeons, when hemostasis may have been undermined during the initial approach. Careful inspection of the muscles under the retractor is necessary as injury can be sustained during the robotic sequence.
Ropivacaine is injected in the wound to facilitate postoperative pain management. No drain is required (no risk of hematoma airway compression as any bleeding seeps into the transaxillary surgical workspace).
Wound closure is done as follows: Vicryl 3-0 suture of the muscle at the level of the clavicle limits dead space in the wound. Skin closure combines Vicryl 3-0 interrupted deep suture and Monocryl 3-0 subcutaneous continuous suture.
RESULTS
Transaxillary procedure was undertaken in 849 patients from 2010 to 2018. Overall complication rate of the transaxillary approach was 2.2% (19 cases), including hematoma, seroma, and transient brachial plexopathy. Hematoma was the main complication (1.5%—13 cases), but none were compressive as they were all located in the transaxillary surgical corridor.
The IJV was injured once (0.12%) and EJV 14 times (1.65%) over the studied period. Bleeding was controlled using surgical clips and did not induce postoperative hematoma.
Concerning the robotic thyroid dissection, complications included 48 cases (6%) of transient hoarseness due to unilateral recurrent laryngeal nerve palsy (spontaneous recovery at the six-week follow-up). Transient hypocalcemia was noted in 87 cases (10%, spontaneous recovery at the one-week follow-up). There were five cases (0.1%) of definitive unilateral palsy and three cases (0.1%) of definitive hypocalcemia (3/5 and 2/3 in cancer patients, respectively). There were two chyle leaks, and no esophageal or tracheal perforations.