INTRODUCTION
Bone conduction devices provide access to sound in children who are otherwise unable to wear or gain benefit from conventional hearing aids. They are used to treat conductive and sensorineural hearing losses resulting from a variety of etiologies, such as aural atresia or single-sided deafness. The application of such devices has been limited in part by soft tissue complications, particularly those associated with skin-penetrating abutments.
METHOD
The study protocol was approved by the Research Ethics Board at the Hospital for Sick Children. All devices were purchased by our institution.
Children who were 18 years of age or younger and who lacked sufficient benefit from percutaneous osseointegrated or nonsurgical bone conduction devices were eligible for participation in this study. The first five devices were inserted following case-by-case approval through Health Canada’s special access program. The remaining 38 devices were implanted after the OSIA2 was approved for clinical use. The described use of the OSIA2 in children under 12 is considered off-label from the perspective of the Food and Drug Administration. (For the key elements of an OSIA2 insertion in an older child with left-sided microtia and aural atresia, see the supplemental video.)
Device placement and preoperative marking. Placement of the device was carefully determined using a modified silastic model. The silastic model was modified with a 2-mm biopsy punch through the neck of the model to allow for correct positioning of the external component. Considerations for implant placement include positioning the actuator no more than 2 cm posterior to the external auditory canal (EAC) and in line with a line drawn through the outer canthus of the eye to superior attachment of the pinna, which roughly denotes the vertical position of the cochlea. In patients who lack an EAC, device location is estimated based on surface landmarks, such as topography and curvature of the temporal bone as well as location of the mastoid tip, which is underdeveloped in aural atresia.
The thickness of the skin overlying the coil and the receiver–stimulator was measured using a 27-gauge needle. Surgical instructions from the manufacturer suggest reducing skin flap thickness if initially greater than 9 mm for optimal coupling of the internal and external devices. Further details on skin flap reduction techniques are described below.
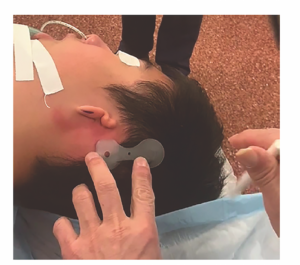
Figure 1. Placement of the device posterior to the predicted location of the pinna and external auditory canal.
Methylene blue was used to identify the location for the implant, as well as the neck of the receiver–stimulator, through the holes in the template (Figure 1). An incision was drawn allowing for at least 1 cm of soft tissue clearance from the perimeter of the actuator. This incision was posteriorly based in the scalp for those with microtia and aural atresia and anteriorly based in the postauricular region for children with a typical pinna.
Implant placement. The implant was then placed using a conical guide drill, followed by a widening drill. In all cases, a 4-mm implant was placed. The dura was often encountered in this young cohort, but there were no dural injuries. Of note, a 3-mm implant is available and could have been placed as an alternative. A bone bed indicator was attached to the implant and rotated to ensure that the bone surrounding the implant was level enough for placement of the OSIA2. Bone polishing using a 3-mm diamond burr was performed if bony clearance was not achieved. A subperiosteal pocket was created for the receiver–stimulator in similar fashion to a cochlear implant. The direction of placement of the device, which ultimately dictates the site of the external component, was guided by the preoperative placement of a methylene blue mark on the neck of the silastic model. The actuator was seated and fixed to the implant. The wound was then irrigated and closed in layers. Postoperative skull radiographs were performed only on the initial five patients in this series.
Management of the thick skin flap. The need for surgical flap thinning should be rarely required in the pediatric population. Children under 7 years of age rarely have a skin flap thickness of more than 3 to 4 mm in this portion of the scalp (Int J Pediatr Otorhinolaryngol. 2020;130:109853). In older children, skin thickness increases with age and with body mass index (BMI) (Int J Pediatr Otorhinolaryngol. 2020;130:109853). Six of the 43 patients had skin flap thickness nearing or greater than 9 mm. In these patients, the coil of the receiver was placed lateral to the temporalis muscle and fascia. In addition to this maneuver, one patient (BMI of 35) underwent concurrent soft tissue reduction. For this patient, the incision was designed to facilitate flap thinning by bringing it to within 1 cm of the neck of the device. In addition, the coil of the receiver–stimulator was placed in the plane overlying the temporalis fascia. Experience with a prior cohort receiving the first generation OSIA device outlines the utility of a separate incision above the coil of the receiver–stimulator to better access the area of the flap to be thinned.
Management of prior implants and devices. Children in our cohort had previously received percutaneous abutments ipsilateral to the planned side of OSIA2 placement. When the goal was to transition from a percutaneous abutment to an OSIA2, the abutment was first removed, and the soft tissues were left to heal over a period of six to 12 weeks prior to OSIA2 placement. In some children, the retained implants approximated the OSIA2 receiver–stimulator and were removed to avoid contact with it. These implants were so osseointegrated that they needed to be drilled out by an otologic drill. With appropriate planning, removal of the prior implant(s), when necessary, can be done at the time of the abutment removal or at the time of the OSIA2 placement. In children with prior percutaneous devices or transcutaneous devices, the incision for OSIA2 was carefully planned to avoid having compromised skin sitting over the actuator while also allowing access for removal of prior implants when required.
RESULTS
The type and etiology of the hearing impairments being rehabilitated with the OSIA2 were heterogeneous but representative of the pediatric population that may seek surgical bone conduction hearing habilitation. Twelve of the 42 children had secondary diagnoses, including four children with Trisomy 21, two with chromosome 18Q deletion, and one child each with Goldenhar, Treacher Collins, Branchio-Oto-Renal, Waardenburg 2E Syndrome, Trisomy 8 and 22, and multiple congenital anomalies.
Mean surgical time was 69 minutes. Soft tissue reduction was required in one child who was obese. There were no subsequent postoperative issues with magnet fitting in this patient. One patient required bone polishing to achieve clearance for placement of the actuator. Surgical complication occurred in two children who experienced irritation at the magnet and incision site due to frequent usage. The soft tissue irritation and mild skin breakdown resolved upon the addition of a magnet soft pad to the external processor.