As with the rest of medicine, the impact of COVID-19 on otolaryngology has been unprecedented, and there are limited scientific data and expert opinions on how to return to practice. Much as we all had to think through patient and practice impact during the shutdown and circumvent negative outcomes when possible, some of those same impact and outcome issues are still at play as physicians consider new protocols.
While the questions are many—Which services to start with? Which patients? Which payers? Which procedures? Which site of service?—otolaryngology practices that include allergic disorder management are definitely aware of the benefits of a steady patient population as they start to rebuild. However, these practices need to consider how best to re-engage their allergy immunotherapy patients.
As otolaryngology practices across the U.S. start to shift from acute and emergent-only to re-opening for elective procedures and full clinics, there are several new issues to consider. Recently, the American Academy of Otolaryngic Allergy (AAOA) leadership drafted some guidance on resuming immunotherapy. The AAOA sought to incorporate practical clinical considerations, including patient and medical staff protection.
Protecting Patients
Restarting subcutaneous immunotherapy (SCIT) after a prolonged absence can result in a serious systemic adverse event, so safety is paramount when re-starting injections. Dosing can be reduced to allow for more gradual re-introduction. A key factor in determining how far back to reduce the dose is the time since the last injection. There are limited data on an exact dosing scheme. In its guidance, AAOA’s example (see below) is in line with expert opinion.
Where a patient is in SCIT also plays a role in determining the dose. Patients in the maintenance phase are generally able to tolerate a higher dosing schedule than those in the escalation phase.
Clinical features should also be examined for each patient—allergic disease severity, prior systemic reactions, time of year for pollen allergies, health status, including medications, and asthma severity and control all need to be considered. Patients who are at increased risk of an adverse reaction due to any of these factors may need a dosing decrease at the first injection beyond what the example describes. The escalation schedule will similarly depend on individual factors and prior injection tolerance. Vial testing prior to the first injection can also provide a measure of safety to determine an individual patient’s reaction and tolerability before proceeding.
Restarting subcutaneous immunotherapy after a prolonged absence can result in a serious systemic adverse event, so safety is paramount when re-starting injections. Dosing can be reduced to allow for more gradual re-introduction.
In addition to SCIT, allergy patients may benefit from corticosteroids, and current recommendations advocate for continued use of inhaled steroids and nasal steroid sprays to maintain a healthy airway and avoid the need for emergency care. Systemic corticosteroids have a special consideration in the era of COVID-19 infection, however—their use in patients with active COVID-19 infections has been shown to worsen the infection in its early phases and should be avoided except in cases of acute respiratory distress syndrome (ARDS). Corticosteroids can still be used in the COVID-19 era. The decision for the use of short bursts of systemic corticosteroids should be made using the shared-decision model, informing patients of the risks of potentially worsening their COVID-19 infection while helping them avoid an emergent case where there could be exposure to COVID-19-positive providers and patients.
Protecting Yourself
As restrictions lift in different parts of the country, while incidence and exposure continue to be monitored, consideration of which protocols will be implemented for patient and staff safety are vital, and use of personal protective equipment (PPE) is of paramount importance. When resuming SCIT injections, providers should wear gloves, gown, mask, hair covering, and eye protection. The adequacy and specific type of PPE to be used during SCIT administration depends upon hospital and government recommendations as restrictions are lifted.
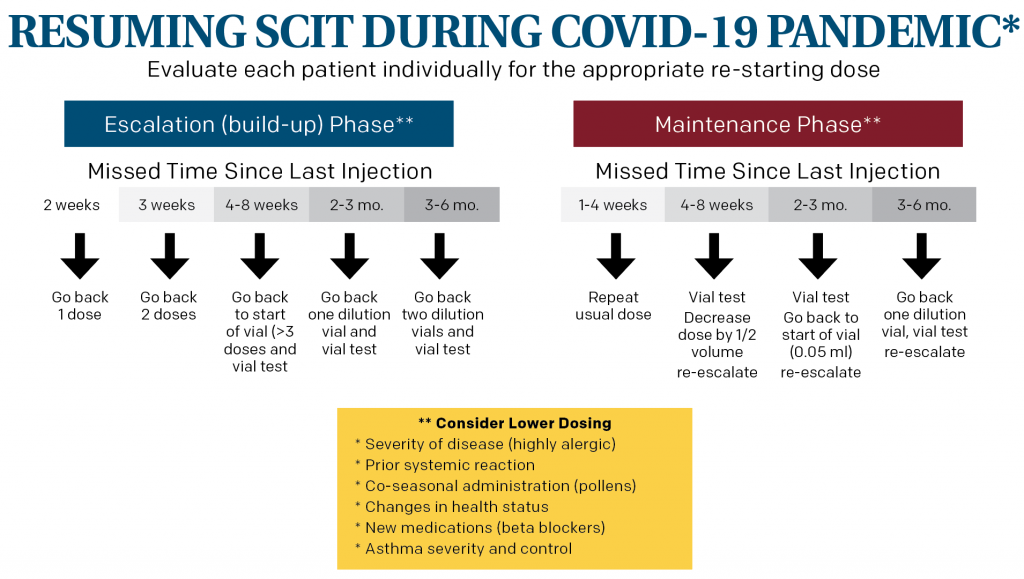
* This example is based on expert opinion as there is a lack of scientific data. Treatment of individual patients will require analysis of their medical condition and individual dosing for immunotherapy.
COVID-19 is transmitted through the aerosolization of viral particles, a fact that’s particularly concerning in allergy management. Use of pulmonary function testing, peak flow testing (PFT), and nebulized medications can promote the aerosolization of viral particles. Whenever possible, these procedures should be deferred or replaced with alternatives—for example, inhaled corticosteroids can be delivered by metered-dose inhalers rather than nebulizer, chest auscultation can be performed rather than PFT, and PFT may even be deferred until COVID-19 restrictions are eased. Frequent surface disinfection with 70% ethanol should be part of an allergy practice’s protocol.
A practice must also consider how it will manage patients and maintain social distancing as it reopens. Limiting additional family members, reducing shared materials such as magazines, pens, or check-in kiosks, and wider seat spacing in waiting areas are some issues to consider.
Patients and staff should be regularly monitored for COVID-19 infection symptoms. Prescreening from the time of booking until the time of entry into the office should be considered. Screening questions should ask about exposure to COIVD-19, cough, shortness of breath, fever, and anosmia. Temperature monitoring can also be implemented prior to entry into the clinic. Patients displaying COVID-19 infection signs and symptoms should be referred to primary care for appropriate testing, when possible, rather than receiving their SCIT injections. All these precautions and more must be a significant part of the decision to reopen.
Dr. Alpen Patel is the 2019-2020 president, Dr. Michael Platt is the treasurer, and Dr. Doug Dawson is the socioeconomic chair of the AAOA.