INTRODUCTION
First described by Singer and Blom in 1980, tracheoesophageal puncture (TEP) with voice prosthesis placement has become the gold standard for voice restoration after total laryngectomy. Voice prostheses have a variable device lifespan and require frequent exchange, typically in the office setting. With the introduction of different models of voice prostheses, new techniques have been described for voice prosthesis placement. The original description of voice prosthesis placement was anterograde, with the passage of the prosthesis from the tracheal side into the puncture tract until the esophageal flange passed into the esophageal side. Currently, the instructions for use for in-office voice prosthesis exchange provided by the major manufacturers of voice prostheses recommend removal of the existing prosthesis followed by anterograde placement of the new prosthesis, with either a dissolving gel-cap or insertion device provided by the manufacturer. This is an effective technique for most patients; however, some patients have a tortuous or highly collapsible TEP tract that makes anterograde insertion of the new prosthesis
very challenging.
We have described a technique for outpatient retrograde voice prosthesis placement that has become a commonly used and successful technique for challenging prosthesis placements in our clinic. Occasionally, this technique can fail for two reasons: a tortuous tract prevents catheter placement, or the catheter cannot be effectively passed in a superior direction to present in the mouth. To overcome these obstacles, we developed and presented our updated technique, which allows for reliable voice prosthesis exchange in patients with known challenging TEP tracts by using the preexisting prosthesis as a guiding conduit.
METHODS
Equipment Required: The new voice prosthesis, an 8 or 10-Fr Dover red rubber catheter with a rounded tip (Cardinal Health Inc., Dublin, Ohio), a 2-0 silk suture, needle driver, scissors, two curved hemostats, suction, and adequate light are necessary for this technique.
Technique: As most patients undergo prosthesis change for valve incompetence or prosthesis age, the existing prosthesis should be in an appropriate position between the trachea and the esophagus. This is confirmed with phonation. The patient is seated upright in the office examination chair. Topical analgesia may be used but is not essential. The position and trajectory of the existing voice prosthesis are examined. If necessary, the existing prosthesis can be rotated to facilitate a superiorly oriented trajectory. The lumen of the prosthesis is suctioned free of any debris. Without removing the existing prosthesis, an 8 or 10-Fr red rubber catheter with a rounded tip (preferably one of intermediate stiffness) is then passed into the lumen of the existing voice prosthesis using a curved hemostat. Care is taken to direct the tip of the catheter cephalad, decreasing the likelihood that the catheter will travel distally down the esophagus into the stomach. If there is significant resistance to the passage of the catheter through the existing voice prosthesis, one can use a narrower catheter or lubricate the catheter with a water-based lubricant. Oil or petrolatum-based lubricants should be avoided to prevent damage to the silicone of the prostheses. If persistent resistance is noted, then the procedure should be aborted. As the catheter is advanced, its tip becomes visible in the back of the patient’s mouth. It is grasped with a clamp, and the tip is advanced out of the mouth. We have not been limited by the patient’s gag reflex during this portion of the procedure, although we do make every effort to limit the duration of this discomfort.
Because using a tract sizer is challenging in patients with tortuous TEP tracts, the size of the new prosthesis is based on prior prosthesis size, patient anatomy, and history. The tab of the new voice prosthesis is secured to the catheter tip with a 2-0 silk suture placed through the tab and the catheter. This is tied circumferentially. The appropriate orientation of the prosthesis is verified (the esophageal flange should
be distal).
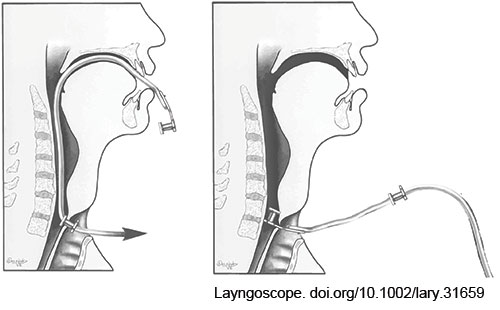
FIGURE 1: Modified retrograde prosthesis placement technique. After passing the catheter through the existing prosthesis in the TEP tract and delivering the tip up through the patient’s mouth, the new prosthesis is secured to the catheter tip and pulled retrograde down the neopharynx and into the TEP tract.
The preexisting prosthesis is now pulled from the tract over the placed red rubber catheter. The new prosthesis can now be delivered in a retrograde fashion by pulling the red rubber catheter back out of the tracheal aspect of the TEP tract (Fig. 1). This will deliver the end of the catheter and the tab of the prosthesis through the tract and into the stoma. Suctioning of mucus or secretions during this process may be necessary. Once the tab of the new prosthesis is visible from the tracheal side of the TEP tract, it is grasped with a hemostat. Another curved hemostat is then used to gently grasp the edges of the tracheal flange of the new prosthesis, adjusting the flange until its entire circumference is freed from the tract. The silk suture connecting the red rubber catheter is cut, and the catheter is discarded.
The new prosthesis is now checked to confirm the appropriate position and function. The prosthesis should rotate easily using a hemostat, confirming that the esophageal flange is properly oriented. The patient is instructed to manually occlude their stoma and phonate to confirm satisfactory function. If rotation is not possible and phonation unsuccessful, this may be due to entrapment of the esophageal flange within the tract, and a longer prosthesis may
be necessary.
RESULTS
Because of the referral nature of our practice, we use this retrograde technique two to four times per month, with 20 retrograde placements in the past year. Three (15%) of these placements were in patients with flap reconstruction, 12 (60%) in patients with a history of esophageal strictures requiring dilation, and two (10%) in patients with a history of stomal stenosis. We have had no complications, and this technique has been successful in all cases where the preexisting prosthesis can be oriented superiorly. We do not use this technique if the preexisting prosthesis cannot be oriented in this fashion.