INTRODUCTION
Surgically implanted bone conduction hearing devices come in three main categories: percutaneous, passive, and active transcutaneous devices (Audiol Res. 2021;11:207–219). The Cochlear OSIA 2 System is one of the most recent active transcutaneous bone conduction hearing devices. It is made up of the OSI200 implant, which contains a coil, magnet, and actuator fixated to the titanium BI300 implant (Cochlear Osia OSI200 Implant: Physician’s Guide). The OSIA 2 System is indicated for patients with conductive or mixed hearing loss with bone conduction pure tone average thresholds of ≤55 dB hearing level or single-sided deafness. The OSIA 2 System reduces the risk of signal attenuation and the skin complications, which were limitations of previous percutaneous and older transcutaneous devices (Cochlear Osia OSI200 Implant: Physician’s Guide). The authors present their surgical modifications to the placement of the OSIA 2 System with a minimally invasive technique that incorporates a bi-leveled small horizontal incision instead of the C- or J-shaped traditional incision, with minimal dissection and endoscopic-guided placement of the fixation screw. This modification shortens the operative time, and in our hands leads to less postoperative pain, intraoperative bleeding, and better esthetics. The technique is hereby described for the first time in children and we have implanted children below the 12 years limit currently set by the FDA.
METHOD
Following ethical review board approval, we describe our approach and present a prospective pilot study of seven children with aural atresia who underwent a minimally invasive OSIA implant surgery (MOSIA). Outcomes reported were postoperative pain, numbness, surgical site infection, wound dehiscence, device exposure, patient satisfaction, and complications. The Wong–Baker faces pain scale designed by the International Association for the Study of Pain and/or the Face, Legs, Activity, Cry, Consolability pain scale were used by certified nurses in the post-anesthesia care unit to assess pain depending on age and development.
Surgical Planning
The OSI200 implant template was modified by creating a hole in the template’s neck with a 2-mm punch biopsy tool, 1.5 cm from the center of the pre-existing hole in the template. The template is then used to outline and mark the desired implant site. We followed the manufacturer’s recommendation and aligned the implant with the external auditory canal, taking care not to interfere with the pinna and placement of glasses (Cochlear Osia OSI200 Implant: Physician’s Guide). In children with microtia, the implant site was outlined approximately 5–6 cm from the presumed ear meatus to preserve the skin for future ear reconstruction. The skin thickness at the magnet location is then measured with a 25 g hypodermic needle and forceps, with a maximum thickness of 9 mm recommended for excellent magnet retention (Cochlear Osia OSI200 Implant: Physician’s Guide).
To prevent device exposure in case of wound breakdown, we injected methylene blue to mark two separate points: the implant site and the newly formed hole in the template’s neck. These markings serve to delineate two separate plane dissection levels, namely the skin level and the muscular/periosteal level. We believe this modification is important in decreasing the risk of implant exposure in case of dehiscence.
Incisions and Biplane Dissection Modifications
We used a transverse skin incision over the implant site rather than the linear, J-shaped, or posterior C-shaped incisions recommended by the manufacturer (Cochlear Osia OSI200 Implant: Physician’s Guide). The transverse skin incision allows for easier access and reduces dissection. We introduced the biplane dissection, subcutaneous and muscular/periosteal, at two different levels to prevent device exposure. The two methylene blue markings that were applied earlier assisted in identifying the two levels during dissection.
Prior to skin sterile prepping and draping, we discussed with the patient and parents about hair removal to ensure proper surgical exposure and good hair cosmesis. With our new incision, we tend to limit the shaving to around the skin incision site. Some cases have also been performed without shaving the hair.
After sterile draping, the skin incision was made, down to the muscular layer and the skin flap is elevated. A separate level linear incision was then made in the muscle down to bone at the level of the second methylene blue marking made at the template’s neck (1.5cm above the previous marking). The muscle incision was then made using monopolar electrocautery until it reached the bone, and the subperiosteal pocket was raised. If the skin thickness is greater than 9 mm, the magnet and coil part of the device can be positioned lateral to the periosteum, which reduces subperiosteal pocket dissection and allows for easier skin flap thinning. Because the OSIA 2 device is screwed to the titanium implant in the skull, the implant does not need to be placed in a tight pocket like a cochlear implant.
Implant Placement
After appropriate dissection, the implant site was marked on the bone using the sterile template. Drilling was performed according to the manufacturer’s instructions, beginning with a 3-mm then a 4-mm conical guiding drill, then a widening drill with countersink, and finishing with the placement of the BI300 osseo-integrated implant (Cochlear Osia OSI200 Implant: Physician’s Guide). It is important to drill perpendicularly to the bone to avoid uneven actuator seating. The drill was set at 2,000 rpm for the drill guide followed by widening, and 40–50 Ncm for implant placement (Cochlear Osia OSI200 Implant: Physician’s Guide).
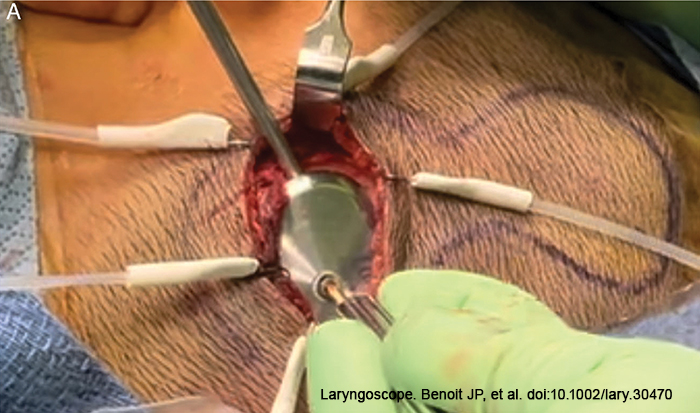
Following implant placement, the bone bed indicator was used to check the surrounding bone to decide whether further drilling was required. The OSI200 was then placed with the coil end in the subperiosteal pocket or lateral to the periosteum, and the actuator portion was screwed to the BI300 implant and hand tightened to a torque of 25 Ncm. Because the actuator is not transparent, it is frequently difficult to align the screw into the BI300 implant due to the nature of the modified small transverse incision. We modified this step using a 30- or 70-degree telescope to endoscopically align the fixation screw into the osseo-intergrated implant. (Figure 1).
Closure
The biplane dissection at two different levels was intended to reduce the risk of device exposure. The muscular/periosteal layer was closed first, followed by the deep subcutaneous layer, and finally skin closure. Mastoid dressing was applied at the end of the procedure.
RESULTS
A total of seven children with aural atresia received MOSIA. One patient had intraoperative sigmoid bleeding, which was controlled with bone wax and changing the implant site. Our youngest patient was 6 years old, and the dura was encountered following the 3-mm conical guiding drilling with no signs of cerebrospinal fluid leak, and a 3-mm implant was inserted. One patient had a partial dehiscence at the skin incision level one week after the surgery, which healed spontaneously with no device exposure, thanks to the biplane approach, as the deeper muscular layer covering the implant was intact.
One month after surgery, all wounds healed nicely with no dehiscence or device exposure. There were no reports of permanent numbness. The children and their families were satisfied. All patients, including the 6-year-old child, had their devices activated one month after surgery with no complications.