Introduction
Empty nose syndrome (ENS) is an uncommon disease characterized by a debilitating spectrum of symptoms such as paradoxical nasal obstruction, nasal dryness, nasal burning, and, for some patients, a withering sense of suffocation (Int Forum Allergy Rhinol. 2017;7:64–71). ENS has been strongly associated with aggressive nasal airway surgery, and, in particular, loss of inferior turbinate (IT) tissue volume (Int Forum Allergy Rhinol. 2017;7:64–71). A promising emerging intervention to reduce the symptomatic burden of ENS in affected patients is a procedure that we have termed the inferior meatus augmentation procedure (IMAP). With IMAP, we attempt to replace the missing tissue of the IT using fashioned cadaveric rib cartilage placed as an implanted graft in the site of the resected or retracted tissue in the lateral nasal airway. In the majority of patients suffering from ENS symptoms, there is typically insufficient IT bone remaining in this region to accommodate placement of a bulky tissue graft; therefore, the inferior meatus and inferolateral nasal wall become the next-best regional site to recreate the rounded, tubular contour and position of the native turbinate organ. The IMAP technique has demonstrated favorable long-term outcomes for this disease entity that has historically been difficult to manage and/or been deemed untreatable, with significant reductions in patient-reported symptoms as measured by the Empty Nose Syndrome 6-Item Questionnaire (ENS6Q) (Laryngoscope. 2021;131:E2736–E2741) and normalization of nasal airflow patterns as demonstrated by computational fluid dynamic studies (Int Forum Allergy Rhinol. 2021;11:902–909). Here we describe the operative and perioperative details for the IMAP.
Method
Preoperative Preparation
Patient selection is of utmost importance to ensure optimal surgical out – comes. Candidates for IMAP surgery must have a diagnosis of ENS as determined by an ENS6Q score >11 and at least two positive blinded in-office cotton tests in which the ENS6Q score improves by at least seven points each time (Int Forum Allergy Rhinol. 2017;7:64–71; Laryngoscope. 2017;127:1746–1752). Patients may also be candidates if they have had a positive response and symptomatic improvement following inferior meatus submucosal filler injection (Int Forum Allergy Rhinol. 2019;9:681– 687). Preoperative evaluation should also serve to optimize management of possible concurrent diagnoses, including trigeminal nerve dysfunction and psychiatric comorbidities, with appropriate referrals considered. Patients must also undergo a standard medical evaluation to ensure they are acceptable candidates for general anesthesia.
The surgery can be undertaken in an outpatient setting. The procedure is performed using standard equipment for endoscopic sinonasal surgery. Additional useful instruments to obtain are a 7200 Beaver blade for the mucosal incision, a #3 Rhoton or duckbill elevator for flap elevation, and #22 blades for rib contouring. The allograft material is irradiated cadaveric rib, which is routinely used for other indications (i.e., nasal reconstruction) and requires storage in a –80°C freezer.
Operative Technique
Visualization can typically be performed with a standard 0- or 30-degree endoscope for all aspects of the IMAP procedure. The anterior and central aspects of the left and right inferior meatuses are injected with 1% lidocaine with 1:100,000 epinephrine using a 25-gauge needle. Injection is meant to induce vasoconstriction as well as hydrodissect the adherent inferior meatus tissue from the underlying maxilla bone.
Following this, the inferior meatal pocket is elevated (see supporting video). A 7200 Beaver blade is used to make a curvilinear incision at the piriform aperture edge just before the axilla attachment of the ITs. The piriform aperture edge typically sits within 1 to 2 mm posterior to the mucocutaneous junction. Hemostasis is facilitated by the use of quar – ter-inch by quarter-inch micropledgets soaked in 1:1,000 epinephrine. A Rhoton #3 or duckbill elevator is then used to elevate a full-thickness, submucoperiosteal flap, creating a sizable pocket between the inferior meatus mucosa and the lateral nasal wall bone. This pocket is elevated posteriorly to a length of about 3.0 to 3.5 cm, or just proximal to the hard:soft palate junction. Importantly, limited dissection of the adjacent nasal floor is performed, such that the dome of the implant projects medially from the bony sidewall into the airway to simulate normal turbinate architecture rather than the implant sinking medially toward the nasal floor and crowding the airway. Meticulous hemostasis of the submucoperiosteal pocket is important to obtain at this point, and can be achieved with topical hemostatic agents such as epinephrine pledgets or thrombin-infused hemostatic matrix.
Intact, cylindrical cadaveric rib graft (>3.0 cm in length) is then thawed in dilute betadine/saline solution and prepared for implantation on a sterile cutting board. Using a #22 blade, the rib is bisected into two half cylinders, one for each side. Then the graft is whittled down in all dimensions to maintain the half-cylinder shape that will fit within the pocket dimensions required for each ENS patient. Typically, one implant measures 2.5 to 3.2 cm in length, 5 to 6 mm in width, and 6 to 8 mm in height in the setting of IMAP following near-total turbinectomy.
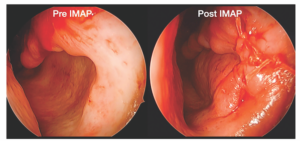
Figure 1. Placement of properly sized, cadaveric rib implant within the submucoperiosteal pocket to augment the left inferior meatus and recreate the rounded, natural contour of a native inferior turbinate projecting from the lateral nasal wall. The incision is then closed using 4-0 Vicryl suture; of note, a 1 to 2 mm airspace between the septum and IMAP graft is maintained for nasal breathing.
The fashioned graft is placed into the inferior meatus submucoper – iosteal pocket (Figure 1). Insertion can be facilitated by retracting the orifice of the pocket during endoscopy, while an assistant advances the half-cylinder implant using forceps. For most patients, the goal is to provide appropriately contoured bulk of the lower nasal airway that projects medially from the inferior meatus sidewall and just underneath any remnant shelf of the IT (if present), while preserving a 1 to 2 mm central nasal airway. In addition, the graft should fit comfortably without excess stretch on the mucoperiosteal flap to prevent flap ischemia or inability to close the incision. Care should be taken to preserve Hasner’s valve during the elevation and implantation to avoid postoperative epiphora. The anterior incision is then closed with interrupted 4-0 Vicryl sutures. Meticulous hemostasis is ensured on the final endoscopic assessment, as typically packing is not placed in the nose.
Results
The patients are discharged home on the day of surgery. Patients are prescribed 10 to 15 oral opioid tablets for pain, as well as two weeks of antibiotics. Patients are also prescribed saline nasal sprays for use two to three times daily, as well as mupirocin ointment to be applied to the anterior nares twice daily. Large-volume rinses are avoided for two weeks.
Patients have their first postoperative visit in the clinic two weeks following surgery. Patients undergo light endoscopic debridement and assessment of the implant sites for tissue dehiscence, cartilage exposure, and graft displacement. Following this visit, patients are typically reassessed at two to three months and six months postoperatively, where graft healing, as well as changes in ENS symptoms using the ENS6Q, are assessed.