INTRODUCTION
Arytenoid dislocation is a rare clinical condition that is often secondary to the tracheal intubation performed during general anesthesia, or occasionally from exogenous blunt contusions to the larynx (Laryngoscope. 2011;121:142–146). Patients often present with hoarseness, painful swallowing, accidental aspiration, dysphagia, and wheezing, and treatment is frequently delayed due to misdiagnosis as recurrent laryngeal nerve palsy (JAMA Otolaryngol Head Neck Surg. 2014;140:1045–1050). Currently, it is commonly accepted that early diagnosis and timely and effective joint reduction are key in treating arytenoid dislocation (J Voice. 2021;35:463–467). However, there is still no consensus about optimal reduction devices and standardized reduction after reduction surgery. In this article, we report a novel reduction device and standardized reduction technique for patients with arytenoid dislocation.
Explore This Issue
October 2023METHOD
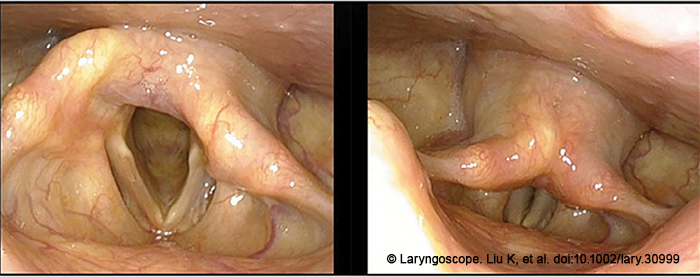
Thirty-three patients were enrolled in this study from August 2019 and May 2022. Patients with arytenoid dislocation diagnosed via medical history, fiberoptic laryngoscopy, stroboscopic laryngoscopy (Figure 1), thin-slice high-resolution laryngeal CT scan and cricoarytenoid reconstruction, and laryngeal electromyography (EMG, bilateral thyroarytenoid, and cricothyroid muscles) were included in the study. Patients underwent stroboscopic examination and voice function evaluation before and after the surgery. Stroboscopic examination was used to determine the position and shape of the arytenoid cartilage, the symmetry of the vocal process, the amplitude of vocal cord vibration, the amplitude of mucosal waves, and the closure of the glottis. Voice function was evaluated using subjective and objective assessments. The subjective voice assessment utilized the overall voice performance Grade (G) in the (Grade, Roughness, Breathiness, Asthenia, Strain) scale from the Japan Society of Logopedics and Phoniatrics. The overall voice performance Grade (G) was graded from 0 to 3, where 0 is a normal voice and 3 is a dysphonic voice. The objective voice assessments included measurements of the jitter index, maximum phonation time (MPT), and dysphonia severity index (DSI) using DIVAS voice analysis software.
Operative Technique
Surgical Equipment. An independently designed joint reduction clamp characterized by a 35.0 cm long, double-jointed clamp body with a curved tip. The tip of the clamp was a hollow triangle that was 1.2 cm in length, with an outer diameter width of 0.7 cm and an inner diameter width of 0.5 cm. The surgical procedure also required a anesthesia visual laryngoscope.
Reduction Method. As shown in the supplemental video, the patient was placed under intravenous general anesthesia with spontaneous breathing. The surgeon held a visual laryngoscope in the left hand to elevate the tongue root and the epiglottis to expose the arytenoid cartilage and posterior commissure bilaterally. Under the direct visualization of the visual laryngoscope, the reduction clamp was inserted through the mouth.
Step 1: Holding. Once it reached the superior edge of the arytenoid cartilage on the affected side, the jaw of the clamp was opened to gently grab the anteromedial and posterolateral sides of the arytenoid cartilage. Caution was taken to avoid excessive force to prevent arytenoid cartilage fracture or iatrogenic joint injury.
Step 2: Returning. The arytenoid cartilage was pushed parallel to the opposite direction of dislocation (posterolateral or anteromedial) and slightly overdrawn by 3 to 5 mm, based on the dislocated direction and angle of arytenoid cartilage determined by the preoperative laryngoscopy and CT scan, and with reference to the normal position of the arytenoid cartilage on the unaffected side.
Step 3: Rocking. We held the arytenoid cartilage and gently rocked it three to five times along the normal joint motion path to release the joint.
Step 4: Rotating. The arytenoid cartilage was held and rotated inside and outside, while centered in the vertical axis of the cone-shaped arytenoid cartilage, with an amplitude of less than 60 degrees.
Step 5: Sliding. The tip of the clamp was slid back and forth from the anterolateral to posteromedial sides along the curved superior edge of the arytenoid cartilage, with a distance less than 1.0 centimeter.
The above five sequential steps were repeated three times. Patients were re-evaluated via visual anesthesia laryngoscopy. If the shape and position of the affected cricoarytenoid joint were symmetrical to the unaffected side and both sides of the vocal processes were at the same level, the patient was directly referred for subsequent voice training. If the cricoarytenoid joint was still asymmetrical between the affected and the unaffected sides under anesthesia and the vocal processes were not at the same level, the patient received the same surgery one week later.
Postoperative Treatment. After the surgery, patients received nebulization twice a day for 3–5 days. Voice training was initiated on the first postoperative day. The efficacy of the treatment was evaluated one month after surgery.
RESULTS
A total of 33 patients with arytenoid dislocation were included in the study. All the patients underwent fiberoptic laryngoscopy, stroboscopic laryngoscopy, and EMG, including 31 (93.9%) cases of anterior dislocation and two (6.1%) cases of posterior dislocation, all accompanied by poor glottic closure. Laryngeal EMG showed intact neuromuscular function in 29 (87.9%) cases and mild functional abnormalities in 4 (12.1%) cases. Preoperative CT scan with cricoarytenoid reconstruction was performed in 25 patients, and 20 (80.0%) of them had abnormal positioning of the arytenoid cartilage, with varying degrees of widening of the cricoarytenoid gap. Five (20.0%) patients did not show direct signs of arytenoid cartilage dislocation in the axial CT images, but the abnormally located arytenoid cartilage was identified in the coronal and sagittal planes and three-dimensional reconstruction of the cricoarytenoid joint.
Following the positive diagnosis, patients underwent the fine and sequential reduction procedure under intravenous general anesthesia and received voice training for one month after the surgery. Among them, 9 (27.3%), 20 (60.6%), and 4 (12.1%) patients underwent the reduction surgery once, twice, or three times, respectively. Among the 28 patients whose arytenoid dislocation resulted from tracheal intubation and general anesthesia, 10 patients’ voices returned to normal on the first postoperative day. Eighteen patients had their voices normalized after one month of voice training. Nine patients showed significant improvement compared with the preoperative day but did not experience complete recovery. In five patients whose arytenoid dislocation did not result from tracheal intubation and general anesthesia, one patient’s voice normalized on the first postoperative day and four patients’ voices normalized after one month of voice training. In patients with or without tracheal intubation and general anesthesia, one patient in each group had G3 voice function preoperatively and recovered to G2 on the first postoperative day, and remained at G2 after one month of voice training.
DSI, MPT, and Jitter indexes were significantly improved on the first day and at the end of the first month after the surgery (p < 0.05). There was also a significant improvement in voice recovery at the end of the first month compared with the first day postoperatively based on the G classification assessment (p = 0.007). The interval between the arytenoid dislocation and the surgery ranged from two to 730 days, with a median of 13 days. Seventeen (51.5%) patients underwent reduction surgery within 13 days of dislocation. Their voice recoveries were significantly better than those who underwent the surgery beyond 13 days of dislocation (p = 0.002). However, age, gender, etiology, type of dislocation, and number of times receiving reduction surgery had no significant influences on voice recovery (p > 0.05).