When considering cost, Dr. Orlandi said the acid test is if the new technology would be worth paying for if it was for your own family member. For example, there was initial concern about the cost/effectiveness ratio for balloon dilation technology in sinus surgery. According to Dr. Orlandi, adoption of this new technology is resolved, now that evidence showing efficacy is available and CPT codes have been established.
Explore This Issue
May 2015Another example, he said, lies with image guidance for sinus surgery. While once a new technology generating controversy, is now an established practice.
Dr. Devaiah highlighted the importance of a post-market analysis for establishing the benefit and safety, or lack thereof, in a new technology. To address the cost issue, he said, early adopters often have to work with their institutions, practices, and sponsoring companies to mitigate the cost in order to use the technology. He also emphasized the need to tell patients if a device is being used off label or is investigational.
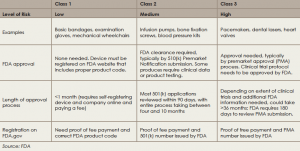
Most medical devices that require FDA approval are class 2 devices deemed to have potentially moderate risk and complexity and are approved through the 510(k) pathway to establish equivalency to a previously FDA approved device. Approval of most of these devices does not establish efficacy; this occurs only after clinical use and post-marketing testing. Otolaryngologists need to be aware of this fact when adopting a new technology in clinical practice, making sure to balance the desire to help a patient by adopting a new technology with a clear understanding of the lack of evidence regarding its efficacy.
Mary Beth Nierengarten is a freelance medical writer based in Minnesota.