Hearing loss is complex, but its effects are relatively straightforward. In adults with late onset hearing loss, progressive decline in hearing can significantly reduce quality of life. In newborns and children with hearing loss, potential negative effects on speech and language development can have long-lasting developmental consequences if not accurately diagnosed and treated early. Because of this danger, newborn hearing screening has been mandated in the United States since 2001.
Explore This Issue
August 2017Contributing to the complexity of hearing loss are the multiple variables in the environment and genetics that cause it. Although mandatory screening has helped to identify newborns with hearing loss, accurate diagnosis of the etiology of the hearing loss remains challenging, given the heterogeneity of the condition. To date, more than 110 genes and 8,000 genetic variants linked to hearing loss have been reported, and more are discovered each day, according to Richard J. Smith MD, a professor in the department of otolaryngology at the University of Iowa Carver College of Medicine in Iowa City.
The importance of identifying the etiology of hearing loss, particularly in newborns and infants, lies in first classifying the condition appropriately to ensure that appropriate treatments are offered. Of the newborns with hereditary hearing loss, most (70%) will have nonsyndromic hearing loss (NSHL), while 30% will have syndromic hearing loss (SHL) (Curr Opin Pediatr. 2012;24:679-686). Distinguishing the type of genetic hearing loss a child has is one critical step to ensuring she receives the appropriate treatment, for her hearing as well as for other comorbidities if the condition is syndromic. For newborns and children in particular, accurate and early diagnosis is critical to providing the appropriate needed interventions that will ensure that these children can develop normally.
Genetic testing is a powerful tool that can augment more traditional diagnostic methods in identifying and diagnosing the etiology of hearing loss. Although available for years, this testing has only recently become a feasible tool in the clinical setting, given new technologies that can provide more comprehensive data. “Genetic testing for hearing loss has emerged over the past decade as the most important first diagnostic test to order in a new evaluation of sensorineural hearing loss,” said Dr. Smith. “After a thorough history, including family history of hearing loss, complete head and neck examination, and appropriate audiometric testing, we recommend genetic testing with a multi-gene testing panel.”
The multi-gene testing panel Dr. Smith refers to is one of the new technologies for genetic testing that has emerged over the past several years. Referred to as next-generation sequencing (NGS) technologies, these tests are replacing older technology that relied on sequencing single genes.
Dr. Smith, who practices at one of the research institutions that provides genetic testing using these NGS technologies, emphasized that all otolaryngologists should know that genetic testing has become an integral part of evaluating a patient with sensorineural hearing loss. “Genetic testing should be considered the first test to be ordered after history, physical exam, and audiometric testing,” he emphasized.
This article provides a brief description of genetic testing using the NGS technologies, followed by recommendations on how to integrate genetic testing for hearing loss in clinical practice. Because most of the current evidence and clinical utility of genetic testing for hearing loss is on newborns and infants, the information presented here is largely in this setting.
Next-Generation Sequencing
Instead of looking for specific gene mutations, modern techniques look at multiple genes simultaneously,” said Stephanie Moody-Antonio, MD, an associate professor in the department of otology and neurotology and director of the cochlear implant program at Eastern Virginia Medical School in Norfolk.
Unlike traditional genetic testing, which isolates individual regions of the genome (most often exons) to identify a single gene for sequencing through a method developed by Sanger in 1977, current NGS technologies use strategies that permit a more comprehensive genetic diagnosis of hearing loss in which all known genes related to the condition can be sequenced simultaneously (Otolaryngol Head Neck Surg. 2015;153:175-182). These technologies use whole-exome sequencing (WES), whole-genome sequencing (WGS), or disease-targeted exon capture strategies that are deemed more suitable for genetic analysis of the heterogeneity of hearing loss (Genet Med. 2014;16:347-355).
Although traditional genetic testing has been useful in identifying hearing loss in which one or a limited number of genes are involved—such as identifying a mutation in the connexin 26 gene, one of the most common forms of genetic hearing loss in children—the heterogeneity of hearing loss requires looking at the broader array of genes and genetic variants that are known to be involved in the condition.
These more comprehensive genetic tests are now available in the United States and worldwide and can be found online at GeneTests, a site that offers genetic testing for a broad range of diseases and disorders, including hearing loss (genetests.org). In the U.S., four research labs offer comprehensive genetic tests for hearing loss (See “Table. 1: U.S. Sites for Comprehensive Genetic Testing for Hearing Loss,”).
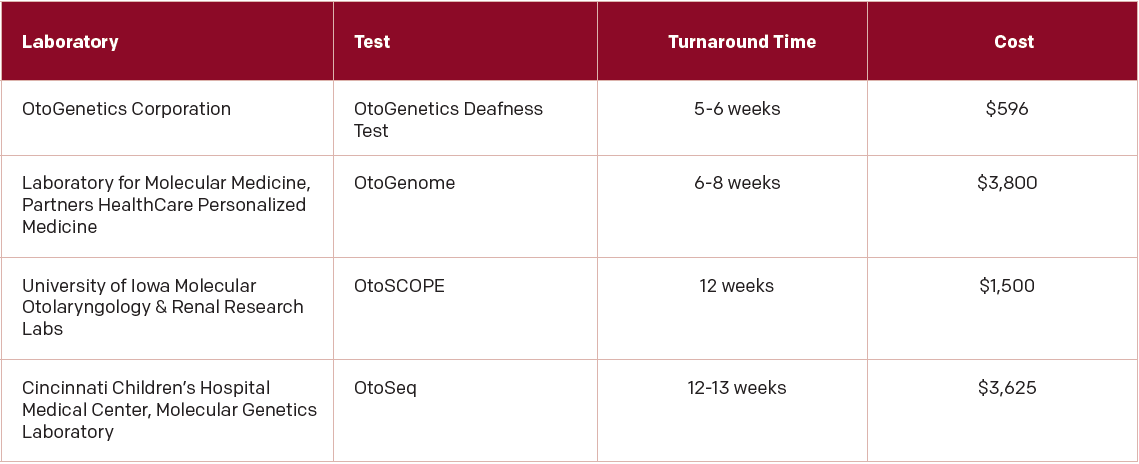
(click for larger image) Table 1: U.S. Sites for Comprehensive Genetic Testing for Hearing Loss
From: Otolaryngol Head Neck Surg. 2015;153:175-182.
The comprehensive genetic testing offered at one of these labs has dramatically improved the diagnostic rate of hearing loss, said Dr. Smith Recently, he and his colleagues published a study looking at the most current evidence on the use of comprehensive genetic testing for hearing loss in the U.S. All four sites in the U.S. use a technology called massively parallel sequencing (MPS), in which hundreds or thousands of gene
regions associated with hearing loss are isolated simultaneously prior to sequencing using a method that relies on targeted genomic enrichment (TGE) (Otolaryngol Head Neck Surg. 2015;153:175-182). Analysis of 20 studies that included 603 patients with unknown causes of hearing loss who underwent genetic testing that used the MPS technology found that this technology provided a better overall diagnostic rate (average 41%) compared with single-gene testing. Overall, the diagnostic rate with this technology ranged from 10% to 83% among the studies.
Given the range of diagnostic rates obtained from the studies, Dr. Smith emphasized that not all comprehensive genetic testing is equal. “Equality can vary at different points along the analysis pipeline,” he said. As such, he said that he and his colleagues “recommend that the testing be comprehensive and that the downstream analysis include an evaluation for copy number variations, with a robust interpretation of the results in the context of the clinical information.” Emphasizing the importance of placing this genetic information in the context of clinical information, he said that he and his colleagues keep an open dialogue with treating clinicians and “always make ourselves available to help.”
The Goals of Genetic Testing
One of the key goals of genetic testing for hearing loss, particularly in children, is to provide a precise diagnosis to guide treatment. This is particularly true in specific situations such as children with the connexin 26 mutation, said Dr. Moody-Antonio. In children with this common cause of nonsyndromic hearing loss, “research predicts that the patient would be very likely to be a successful cochlear implant user,” she said.
A genetic diagnosis of Usher syndrome, another type of hearing loss, may also change how the hearing loss is treated. “In this case, bilateral cochlear implantation and oral language training would be preferred, since the child would eventually lose the ability to communicate with a visual language such as American Sign Language,” she said.
For Rick Friedman, MD, PhD, director of the University of Southern California (USC) Acoustic Neuroma Center and professor of otolaryngology and neurosurgery at USC’s Keck School of Medicine in Los Angeles, one of the key reasons for genetic testing in children is that it is a safer way to determine which children may benefit from a cochlear implant. “Prior to genetic testing, we’d order a CT scan to see if a child would be a good candidate for cochlear implantation or to see if we could find a cochlear anomaly,” he said, adding that it is increasingly recognized that early exposure to radiation by CT scan is probably not good for a child’s brain. “I think genetic testing is helpful because it helps to avoid radiation exposure in some children and gives prognostic information and direction to rehabilitate children.”
Another important goal of genetic testing is to provide genetic counseling for parents who may want information on the chance of having another child with hearing loss, said Dr. Friedman.
Genetic testing also may be used as an adjunct to hearing screening at birth, according to Anil K. Lalwani, MD, vice chair for research and director of the division of otology, neurotology, and skull base surgery at Columbia University College of Physicians and Surgeons in New York City. Although he and his colleagues are not yet using genetic testing in this way, he sees the potential the process has for ensuring that appropriate intervention is not delayed in children who have a true hearing deficit. “Imagine the scenario where the infant who fails hearing screening immediately undergoes genetic testing before discharge from a well-baby nursery,” he said. “If the test is positive, one would know that the likelihood of hearing loss is high, so the next appointment could be about both establishing the severity of hearing loss [and arranging] therapeutic intervention.”
Using Genetic Testing in the Clinic
In recognition of the role genetic testing may play in evaluation of hearing loss, and the advances in technology the field is experiencing, the American College of Medical Genetics and Genomics (ACMG) published practice guidelines in 2014 for the clinical evaluation and etiologic diagnosis of hearing loss (Genet Med. 2014;16:347-355.) Table 2 provides recommendations on when genetic testing may be useful (See “Table 2: ACMG Suggested Use of Genetic Testing for Clinical Evaluation of Hearing Loss”).
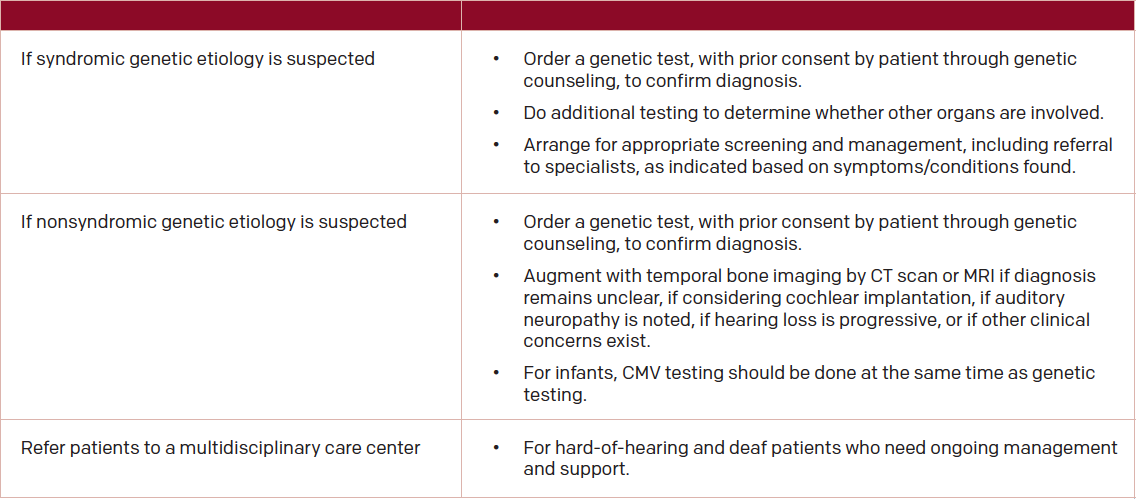
(click for larger image) Table 2: ACMG Suggested Use of Genetic Testing for Clinical Evaluation of Hearing Loss
Source: Genet Med. 2014;16:347:55.
To date, the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) has offered no official guidance to otolaryngologists on the use of this guideline. Input from some of the sources in this article suggests that individual otolaryngologists must use their best judgment to determine whether they should order a genetic test or refer the patient to a geneticist. Dr. Moody suggests that clinicians consider genetic testing frequently and provide it as a typical component to the algorithm to diagnose hearing loss, with emphasis on the idea that clinicians need to develop relationships with local geneticists for patients in whom more extensive genetic counseling is needed.
Although Dr. Lalwani routinely ordered and handled genetic testing in his earlier practice, he said that he now refers patients to a geneticist, given the complexity of genetic testing and the analysis of results. “Genetic testing is so complex that to do justice to it, specifically as we move into sequencing larger segments of our genome and whole genome sequencing, it may be best to refer to a geneticist so that the patient can benefit from their comprehensive consultation,” he said. All agreed that genetic counseling is a critical component to any genetic testing.
Mary Beth Nierengarten is a freelance medical writer based in Wisconsin.
Key Points
- Genetic testing is a powerful tool that can augment more traditional diagnostic methods in identifying and diagnosing the etiology of hearing loss.
- Although available for years, gene testing has only recently has become a feasible tool in the clinical setting.
- Among the key goals of genetic testing for hearing loss, particularly in children, is to provide a precise diagnosis to guide treatment.
- Clinicians consider genetic testing frequently and provide it as a typical component to the algorithm to diagnose hearing loss, with emphasis on the idea that clinicians need to develop relationships with local geneticists for patients in whom more extensive genetic counseling is needed.
Hospitalists as Test Subjects
Genetic susceptibility to otitis media in childhood
Abstract. Otitis media (OM) is a common disease in early childhood characterized by inflammation of the middle ear cavity. Heritability studies suggest that there is a substantial genetic component (40%–70%) to the risk of recurrent acute OM, defined as three or more episodes in 6 months or four or more episodes in a year, or chronic OM with effusion (COME), defined as middle ear fluid for ≥3 months. To date, only a handful of the regions/genes underlying this genetic susceptibility have been identified. These include several regions of linkage on chromosome 3p25, 10q22, 10q26, 17q12, and 19q13 identified by two genome-wide linkage scans, which appear to harbor susceptibility loci. Fine mapping of these regions has yet to identify the causative genes. Several candidate genes studies have also been reported, with candidates selected on the basis of a plausible biological role in OM or through OM mouse models. Reviewed in this article, these studies have identified positive association at 21 genes, including FBXO11, TLR4, and TNF, with association at five of these replicated in independent populations. However, these studies have been based on small sample sizes, and it is only recently that well-powered OM cohorts suitable for genome-wide association studies (GWAS) have become available. Results from such GWAS will identify novel genes involved in this complex disease. Identification of the genes that contribute to OM susceptibility in childhood will provide important insights into the biological complexity of this disease that could ultimately contribute to improved preventative and therapeutic strategies to reduce the incidence of this disease (Laryngoscope. 2012;122:665–675 ).
GJB2-associated hearing loss: Systematic review of worldwide prevalence, genotype, and auditory phenotype
Objectives/Hypothesis. To perform a systematic review of GJB2-associated hearing loss to describe genotype distributions and auditory phenotype.
Data Sources. 230 primary studies identified from Pubmed.
Review Methods. PubMed was searched systematically to screen broadly for any study reporting on genotype and carrier frequencies for biallelic GJB2-associated hearing loss in defined populations around the world. Genotype and audiometric data were extracted and subjected to meta-analysis to determine genotype distributions, carrier frequencies, rates of asymmetric or progressive hearing loss, and imaging abnormalities.
Results. A total of 216 articles comprising over 43,000 hearing-loss probands were included. The prevalence of biallelic GJB2-associated hearing loss was consistent across most of the 63 countries examined, with different mutations being predominant in different countries. Common mutations were found in greater than 3% of the general population worldwide. Meta-analysis of 48 case-control studies demonstrated a two-fold higher carrier frequency among hearing-impaired individuals compared to normal-hearing controls for truncating alleles, but not V37I. Progression, asymmetry, and imaging abnormalities were present in 14% to 19% of individuals with GJB2-associated hearing loss.
Conclusion. GJB2 mutations are highly prevalent around the world. The multiple predominant mutations present in different populations attest to the importance of this gene for normal cochlear function and suggests an evolutionary heterozygote advantage.
The unusually high carrier rate for truncating mutations among hearing-impaired individuals is consistent with either the presence of complementary mutations or a carrier phenotype. The significant rate of asymmetry and progression highlights the importance of diagnostic workup and close follow-up for this highly variable condition. (Laryngoscope, 2014;124:E34–E53).