Reprinted from the August 15 Neurology Today with permission from the American Academy of Neurology.
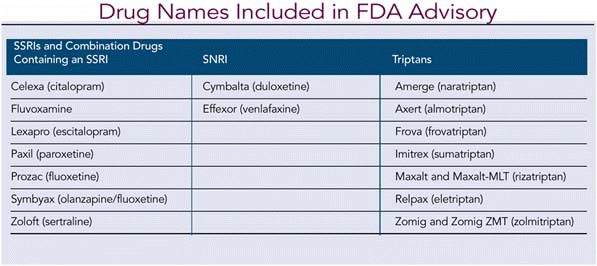
A new Food and Drug Administration (FDA) advisory warns that the combined use of triptans and selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome, which occurs when the body has too much serotonin.
Migraine experts maintain that this risk is small, however, and they do not advocate stopping the combined use of these drugs for people with migraine and the comorbidity of depression and anxiety in the absence of serotonin syndrome symptoms. They do suggest, though, that clinicians monitor and discuss the risk with their patients. (See “Symptoms of Serotonin Syndrome.”)
“I understand the FDA’s obligation to the public safety,” said Stewart J. Tepper, MD, Associate Clinical Professor of Neurology at Yale University School of Medicine and Director of the New England Center for Headache. The risk of a life-threatening event when using these drugs may not be zero; however, migraine and the comorbidity of anxiety and depression are prevalent and people need treatment, he said. “I don’t want to say the serotonin syndrome isn’t serious, but its occurrence in combination is highly unlikely,” he added.
Asked to comment, Crystal Rice, an FDA spokesperson, acknowledged that the risk is small, but she said that “knowing about it may help patients and physicians recognize this event, if it does occur, and may facilitate appropriate management of the patient.”
Data Summary and Advisory
The FDA reviewed 27 cases of serotonin syndrome reported from 1998 to 2002 in association with concomitant SSRI or SNRI and use of triptans. Two reports involved life-threatening events, none fatal, and 13 patients required hospitalization. Some cases occurred in patients who had previously used concomitant SSRIs or SNRIs and triptans without experiencing serotonin syndrome.
The signs and symptoms of serotonin syndrome were highly variable and included respiratory failure, coma, mania, hallucinations, confusion, dizziness, hyperthermia, hypertension, sweating, trembling, weakness, and ataxia.
In eight cases, recent dose increases or the addition of another serotonergic drug to an SSRI/triptan or SNRI/triptan combination were temporally related to symptom onset. The median time to onset subsequent to the addition of another serotonergic drug or dose increase was one day, with a range of 10 minutes to 6 days.
“SSRIs, SNRIs, and triptans independently increase serotonin levels,” wrote the FDA. “Therefore, it is expected that concomitant use of SSRIs or SNRIs and triptans would result in higher serotonin levels than the serotonin levels observed with the use of SSRIs, SNRIs, or triptans alone, potentially leading to serotonin syndrome.”
According to the FDA, physicians prescribing a triptan, SSRI, or SNRI should remember that triptans are often used intermittently and that different physicians may prescribe these drugs. Clinicians should weigh the potential risk of serotonin syndrome with the expected benefit of using the drug combination; discuss the possibility of serotonin syndrome with patients; follow patients closely, particularly during treatment initiation, with dose increases or with the addition of another serotonergic medication; and instruct patients to seek medical attention immediately if they experience serotonin syndrome symptoms.
Serotonin Syndrome Is Rare
Robert Shapiro, MD, PhD, President of the Headache Cooperative of New England and Associate Professor of Neurology at the University of Vermont, estimated the annual incidence of serotonin syndrome to be less than 0.03% of patients also exposed to SSRIs and triptans, and the annual incidence of life-threatening events to be less than 0.002% of those similarly exposed.
“This was a conservative assessment based on a number of assumptions,” said Dr. Shapiro. The estimation reflects the FDA’s 27 cases of serotonin syndrome, representing an assumed 10% of actual cases in the United States, as well as a report by Dr. Tepper (Headache 2003;43:44–48), in which 240,000 patients of the 65 million covered by Merck-Medco insurance from 2000 to 2001 had filled two or more triptan prescriptions within a year. Twenty-one percent of these were also taking an SSRI.
Dr. Shapiro extended the Merck-Medco figures to the 85% of the US population that has health insurance, about 250 million people, and estimated that about 200,000 Americans per year were similarly exposed to both triptans and SSRIs. A number of other assumptions, such as no change in triptan or SSRI usage rates, no FDA cases occurring in uninsured patients, and no contribution of SNRIs to the triptan co-prescription were factored into his analysis.
Do Triptans Play a Role?
Based on available literature, it is still far from clear whether adding a triptan to an SSRI significantly increases the risk of serotonin syndrome above that seen with the SSRI alone, said Dr. Shapiro. Eight of the 27 FDA cases were associated with co-administration of yet another serotonin-active drug along with the triptans and SSRIs, so the contributions of triptans were particularly unclear in these cases, he said.
Dr. Shapiro found no case reports of serotonin syndrome associated with triptans taken alone, whereas the incidence of serotonin syndrome following exposure to SSRIs alone is estimated to be 0.5 to 0.9 cases per 1000 patient-months of treatment (Br J Gen Pract 1999;49:871–874).
Evidence that triptans independently increase serotonin levels is lacking, said Stephen Silberstein, MD, Director of the Jefferson University Hospital Headache Center. To the contrary, one study in rat brain indicates that acute triptans decrease serotonin release (Cephalalgia 2004;24:2–11), he noted. Another study found 22 cases of adverse events associated with fluoxetine (Prozac) as reported to the drug’s manufacturer (Acta Psychiatr Scand 1997;95:551–552); four of these provided evidence of interaction with a sumatriptan to cause the serotonin syndrome, but only two of them showed good evidence, said Dr. Shapiro. Yet another study involving six patients, some of whom were taking lithium and imipramine in addition to SSRIs and triptans, found an association with serotonin syndrome (Cephalalgia 1996;16:323–327), said Dr. Silberstein.
A study that evaluated the tolerability and safety of combining other medications in patients taking subcutaneous sumatriptan found that 1,784 of the 12,339 migraine patients were on SSRIs and subcutaneous sumatriptan during a one-year period, and none experienced serotonin syndrome (Cephalalgia 1999;19:668–675), said Dr. Tepper.
Most experts said that the data indicate that triptans are safe in combination with SSRIs. But it is also true that most headache specialists, including those interviewed for this article, are involved in the commercial promotion or development of triptans, noted Dr. Shapiro. “It’s hard to find an expert who has not been commercially involved in this class of medicines,” he said.
Treatment Will Not Change
None of the migraine experts recommended that the combination of triptans and SSRIs or SNRIs be discontinued unless symptoms arise.
“I say, ‘If you’re feeling intoxicated, feel sick, get clumsy on your feet, start to twitch, then stop taking the medications,’” Dr. Tepper said. He also recommends trying a non-SSRI or non-SNRI alternative for depression or anxiety and continuing triptans for migraine.
Richard Lipton, MD, Director of the Montefiore Headache Unit and Professor of Neurology at the Albert Einstein College of Medicine, agreed that the risks and benefits of treatment should be discussed with patients. “Patients should be told that serotonin syndrome is a rare complication of medical therapy,” he added.
Patients on SSRIs or SNRIs and triptans should avoid other drugs that act on the serotonin system and keep the dose on the lower end of the safe dose range for triptans, explained Dr. Lipton.
The Bottom Line
Overall, the FDA advisory is based on a preliminary data analysis, noted Dr. Shapiro. The fine print of the Healthcare Professional Sheet with FDA Alert states, “FDA is considering, but has not reached a final conclusion about this information. FDA intends to update this sheet when additional information or analyses become available. “The FDA advisory is premature and their fine print is very apt,” Dr. Shapiro concluded.
Symptoms of Serotonin Syndrome
- Restlessness
- Hallucinations
- Loss of coordination
- Fast heartbeat
- Rapid changes in blood pressure
- Increased body temperature
- Overactive reflexes
- Nausea
- Vomiting
- Diarrhea
©2006 The Triological Society


Leave a Reply