INTRODUCTION
Posterior glottic insufficiency (PGI) is a potential sequela of prolonged intubation. Sustained pressure from the endotracheal tube results in ulceration, erosion of the vocal process, and subsequent tissue loss of the medial arytenoid cartilage and mucosa (Ann Otol Rhinol Laryngol. 2017;126:268–273). The resultant keyhole aperture, or trough, in the vocal process of the arytenoid can be unilateral or bilateral and results in air escape during phonation. PGI is distinct from a posterior glottic diastasis, which can be secondary to graft placement following a posterior cricoid split placing the arytenoids further apart.
Clinically, patients with PGI present with a breathy voice, decreased maximum phonation time, and psychosocial stressors related to vocal handicap. Because the arytenoids can obscure the posterior glottis during in-office laryngoscopy, diagnosis may be elusive and the treatment is often challenging (J Voice. 2020;S0892-1997:30261-7). The tight adherence of the mucosa on the arytenoid often precludes injection augmentation. In addition, laryngeal framework surgery often fails to correct the posterior glottic defect. Other described surgeries include autologous mucosal grafts, large aryepiglottic fold rotational flaps, or posterior cricoid reduction laryngoplasty (J Voice. 2020;S0892-1997:30261-7). However, these approaches may diminish airway caliber and may require intubation or tracheostomy during the recovery period. Herein, we describe a novel technique and outcomes for an endoscopic posterior rotational flap for the treatment of PGI.
METHOD
With Baylor College of Medicine Institutional Review Board approval (H-50056), a retrospective review was performed in patients undergoing endoscopic posterior rotation flap for PGI between October 2018 and April 2021 by the senior author. The diagnosis of PGI was established following awake laryngoscopy to confirm vocal fold mobility and adequate closure of the membranous vocal folds and direct laryngoscopy with inspection of the vocal processes. Pre- and postoperative assessments included the Pediatric Voice Related Quality of Life (PVRQOL) questionnaire and the Consensus Auditory Perceptual Evaluation of Voice (CAPE-V) performed by trained speech language pathologists (D.D. and S.H.). Voice samples were recorded and analyzed with Multi-Dimensional Voice Profile and Real Time Pitch. Objective measures included CAPE-V scores, maximum phonation time (MPT), mean fundamental frequency for vowels and consonants, pitch range, jitter, shimmer, noise to harmonic ratio, mean and maximum intensities, and PVRQOL survey results. Postoperative data were collected between one and six months postop.
Surgical Technique
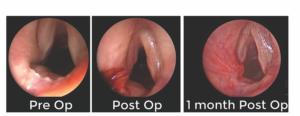
Figure 1. Still images demonstrating a posterior glottic insufficiency with a deep trough (left), immediately postoperative with endoscopic posterior rotation flap sutured into place and filling the trough (middle), and one month postoperatively with a well-healed
rotational flap filling the prior defect (right).
Using a tubeless spontaneous ventilation technique, microsuspension direct laryngoscopy was performed with a Lindholm laryngoscope. A CO2 laser via micromanipulator Digital AcuBlade micromanipulator was used to create a medially based mucosal and subcutaneous tissue U-shape flap along the medial surface of the arytenoid.
Dissection was continued just lateral to the trough with care not to buttonhole the mucosa. A laryngeal flap elevator was used to tunnel a pocket underneath the trough, helping to medialize it. In some individuals, the mucosa was tightly adherent to the arytenoid, and the laser was used to slice a sliver of the arytenoid to create the pocket.
Next, the mucosa is removed off the lateral portion of the flap, using the CO2 laser, to minimize the risk of a postoperative mucocele. The lateral edges of the flap are then folded and tucked deep into the pocket. This packs the trough and fills the posterior glottic aperture. The flap is tacked in a folded position endoscopically with 6–0 VICRYLsuture on a S-28 needle. Key steps of the surgery are included in video format (see supporting video).
If there is bilateral PGI, the procedure can be staged. Concurrent bilateral flaps should be reserved for older patients with adequate airway caliber, whereas the flaps should be sequentially staged in younger children to avoid airway compromise and the need for tracheostomy. Postoperatively, the patient is admitted for overnight observation. No perioperative antibiotics are used, and antireflux medications are not routinely given unless there is a previous diagnosis of gastroesophageal reflux.
RESULTS

Figure 2. Results demonstrating significant improvement in (A) Consensus Auditory Perceptual Evaluation of Voice (CAPE-V)
overall, (B) CAPE-V breathiness, and (C) maximum phonation time (MPT); (D) Pediatric Voice Related Quality of Life (PVRQOL) trended toward improvement without statistical significance.
Five patients with PGI underwent the endoscopic posterior rotation flap, three of which were bilateral. The mean follow-up period between surgery and obtaining postoperative data was 70 days (range 20–160). No adverse events were encountered. In all cases, endoscopic visualization demonstrated significant improvement in PGI (Figure 1). Findings shown in Figure 2 demonstrated significant improvement in CAPE-V overall (P = 0.0066), CAPE-V breathiness (P = 0.0126), and MPT (P = 0.0003). PVRQOL trended toward improvement without statistical significance (P = 0.0945).