For most affected children, tracheomalacia—a congenital deformity of the trachea that causes the airway to collapse with breathing and coughing—is a medically manageable inconvenience that resolves over time. But for some children and families, tracheomalacia is a life-threatening condition that also interferes with child development and family bonding.
Explore This Issue
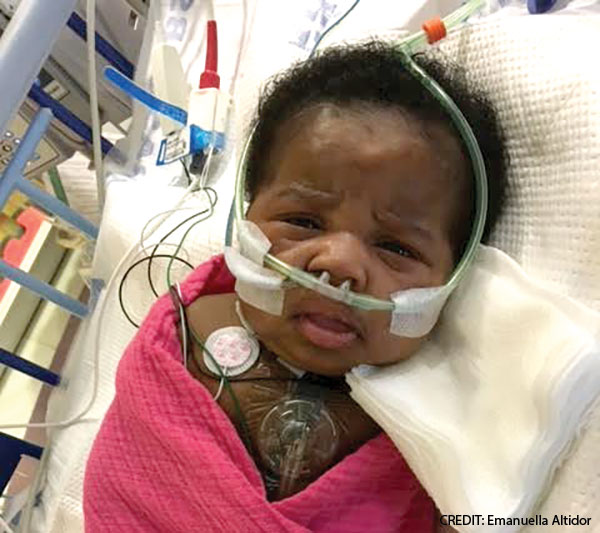
October 2024Justice Altidor was one of those children. Justice and her twin, Journee, were born at 34 weeks gestation because doctors were concerned about twin-to-twin transfusion syndrome, said Emanuella Altidor, the girls’ mother. Justice had also been diagnosed with a double aortic arch while in utero.
“They whisked her away as soon as she came out because they noticed her difficulty breathing,” Ms. Altidor said.
Justice was intubated, connected to a ventilator, and scheduled for cardiac surgery. She underwent surgical repair of the double aortic arch eleven days after birth but continued to have breathing difficulties. Justice had severe tracheomalacia; she was ventilator-dependent and required intensive care. Her parents couldn’t pick her up because the slightest movement could shift her breathing tube out of place and trigger a respiratory crisis.
Not so very long ago, Justice would have likely spent the first years of her life tethered to a ventilator and in and out of the hospital—if she survived. The estimated mortality rate for severe tracheomalacia is 80% (Int J Pediatr Otorhinolaryngol. 2023;169:111559).
“These kids can be so sick that, if the breathing tube moves just a tiny bit, they can experience complete respiratory arrest,” said Kevin Maher, MD, a pediatric cardiologist who is director of the Cardiac Intensive Care Unit at Children’s Healthcare of Atlanta.
But thanks to medical innovation, collaboration, and a few custom 3D-printed, bioabsorbable tracheal splints, Justice was able to go home with her parents and sister—without a ventilator or oxygen—just four months after birth. She was free to crawl, sit, and eventually run. Now four years old, Justice plays soccer, rides her bike, and swims.
The treatment that enhanced Justice’s life isn’t yet widely available. Only five institutions in the U.S. currently offer tracheal splints to support the airway, and the Georgia Institute of Technology is one of only two locations capable of custom printing these devices. Each case must be individually considered and submitted to the U.S. Food and Drug Administration (FDA) for possible approval under expanded access guidelines for lifesaving, cutting-edge therapies. To date, approximately 30 children have undergone surgical implantation of the custom airway splints.
These devices have had a profound impact on the children who receive them, their families, and the medical professionals involved. And, someday, the innovation that led to this transformative treatment may advance otolaryngology.
Managing Severe Tracheomalacia
Tracheomalacia is the most common congenital tracheal defect, with one in 2,100 children born with the condition, according to the National Organization for Rare Disorders. Most children with the condition have a mild to moderate presentation, and symptoms may improve and even resolve by two years of age. Children with severe cases of tracheomalacia may also experience significant improvement as they grow.
 “The vast majority of these kids will actually outgrow it,” said Matthew Brigger, MD, MPH, a pediatric otolaryngologist who serves as director of the Center for Pediatric Aerodigestive Disorders and Airway Surgery at Rady Children’s Hospital-San Diego. “You just have to give it time. Sometimes, you need a tracheostomy to get to that point.”
“The vast majority of these kids will actually outgrow it,” said Matthew Brigger, MD, MPH, a pediatric otolaryngologist who serves as director of the Center for Pediatric Aerodigestive Disorders and Airway Surgery at Rady Children’s Hospital-San Diego. “You just have to give it time. Sometimes, you need a tracheostomy to get to that point.”
Fortunately, few children have severe tracheomalacia. “It’s rare in the sense that you’re not going to see one case a week. But if you’re in a high-volume center, you’ll see two or three a year,” said Steve Goudy, MD, division chief of otolaryngology at Children’s Healthcare of Atlanta. “Tracheostomy and ventilation are the historical answers” that allow children to go home with their families, he said.

Justice, top, received 3D-printed tracheal splints (left), printed by the Georgia Institute of Technology, and now, she and her twin, Journee, are thriving.
Of course, tracheostomies and long-term ventilation aren’t ideal solutions. Tracheostomies require careful care and maintenance; positive pressure ventilation can cause “ongoing trauma to the lungs,” Dr. Maher said. Tracheostomy tubes and ventilators also inhibit children’s vocal and motor development.
To the FDA’s credit, they recognize that getting a device like this fully approved may take 10 years, sometimes longer. So, if there is someone with critical health issues who could potentially benefit from the device, the FDA is willing to review each individual case.” — Kevin Maher, MD D
As a result, physicians sometimes use other approaches. During a tracheopexy, surgeons stabilize the floppy areas of the trachea by securing it to other nearby organs or tissues; these attachments prevent the trachea from collapsing as the affected individual breathes. It’s an effective, but far from ideal, treatment for severe tracheomalacia, as it requires operating around major blood vessels in a small chest cavity.
Some surgeons use intratracheal stents to hold the airway open. Intraluminal metal stents can be inserted endoscopically—a major advantage over open chest surgery. And evidence to date suggests that intraluminal stenting can be effective in children with tracheomalacia. In a 2023 paper describing the results of airway stenting with balloon-expandable coronary bare-metal stents to treat tracheobronchial disease in medically complex infants with congenital heart disease, six of eight treated patients were able to wean from mechanical ventilation within days of stent placement. The stents were removed approximately six months later, and bronchoscopy after stent removal “demonstrated a rounder configuration of the airway consistent with bronchial remodeling” (J Soc Cardiovasc Angiogr Interv. 2023;2:101068).
But Dr. Brigger, one of the authors of the paper, notes that internal airway stents “come with their own set of complications,” including the possible buildup of granulation tissue, which may occlude the airway and erosion of the stent through the walls of the airway.
External airway splints avoid those complications but require open chest surgery. The first report of external pediatric airway splinting using patient-specific 3D-printed splints was published in Science Translational Medicine in 2015. The study described positive results using bioabsorbable custom-printed airway splints to treat three children, ages three months, 16 months, and five months at the time of surgery. All three children had life-threatening tracheobronchomalacia and were confined to the hospital, dependent on mechanical ventilation via a tracheostomy prior to surgery. All three experienced significant improvement in their symptoms; one child no longer required ventilator support and was discharged home just three weeks after surgery (Sci Transl Med. 2015;7:285ra64).
A 2021 study reported on the results of implantation of external moldable, bioresorbable stents in 14 severely symptomatic children ages two months to 14 years. Seven of the children had tracheostomies in place at the time of surgery; six required mechanical ventilation in monitored, inpatient settings. Post-surgery, five patients were discharged home; two died of unrelated causes. Three children still required mechanical ventilation (JTCVS Tech. 2021;8:160-169).
When Justice continued to have breathing difficulties after surgical repair of her congenital aortic arch, Drs. Maher and Goudy and her parents discussed the possibility of supporting her airway via custom-printed 3D splints.
As otolaryngologists, we need to continue to tell the story of the unmet needs of our patients. We need to communicate that to engineers and scientists and work alongside them to develop new therapies.” — Stephen Goudy, MD
“They told us that a tracheostomy is what they normally do, but they also presented the splint as an option,” Ms. Altidor said. “They said she’d be the youngest person ever to have something like this put in and that it would have to get approved by the FDA. Her dad and I really wanted her to have the best quality of life, so as soon as they presented an alternative, we were happy to go with the alternative.”
Collaborating to Create Custom Solutions
A diverse team of healthcare experts, scientists, and engineers worked together to create and implement a tailored solution for Justice. Emory University, Children’s Healthcare of Atlanta, and the Georgia Institute of Technology frequently collaborate to solve important problems in pediatrics and develop technological solutions to improve children’s health, so the people are in place. Scott Hollister, PhD, the Patsy and Alan Dorris Chair of Pediatric Technology and professor of biomedical engineering at the Georgia Institute of Technology, was recruited to Atlanta in 2017 because he’d previously pioneered the development of custom 3D-printed medical devices, including external airway splints, at the University of Michigan.
Drs. Maher and Goudy consulted with Dr. Hollister and other physicians, healthcare professionals, and engineers to devise a feasible plan of care for Justice. The evaluation and planning process included obtaining inspiratory and expiratory CT scans of Justice’s airway to precisely identify and measure areas of obstruction. Engineers and technicians then used that data to design and custom print splints made of polylactic acid (PLA), a bioabsorbable material. (Some children, Dr. Maher said, require multiple splints; others may need just one.)
Together, the team also worked to identify optimal surgical stitch placement, using computer modeling and computational fluid dynamics to virtually test the impact of assorted options.
“I’d give them ideas, and they’d do the computer modeling in the lab. They’d run the program all night and, in the morning, we’d meet and see where there’s airflow and where there would be restriction,” Dr. Goudy said. Technology allowed the team to engage in virtual surgical planning, which helped them minimize surgical time and increase the chances of a successful outcome.
Technology also aided family communication and informed consent. “We can 3D print models of the airway and the splints, so the families can hold them and put the splints on. We also show them some of the computation fluid dynamic modeling we use,” Dr. Goudy said.

Justice can do all the things many four-year-olds can do, like play soccer, thanks to an incredible team of healthcare experts, scientists, and engineers from Emory University, Children’s Healthcare of Atlanta, and the Georgia Institute of Technology.
Because the modeling program uses red to highlight areas of airway obstruction, it’s easy for families to visualize the impact of surgery. Ms. Altidor said she and her husband appreciated the time the team spent explaining the procedure to them. “We felt we were partners,” she said. “We were working together and collaborating.”
The family was concerned about how long it might take to obtain FDA approval. But the process didn’t take as long as they feared.
“To the FDA’s credit, they recognize that getting a device like this fully approved may take 10 years, sometimes longer. So, if there is someone with critical health issues who could potentially benefit from the device, the FDA is willing to review each individual case,” Dr. Maher said. Because time is of the essence, turnaround times can be less than a week.
A challenge in Justice’s case is that she did not have a tracheostomy, the traditional standard of care. “You have to demonstrate that there’s nothing else clinically available that would benefit the patient,” Dr. Goudy said. “So, historically, these implants were only offered to patients who had tracheostomies and still were not doing well. Justice didn’t have a trach, but we didn’t want to put one in.”
Fortunately, the FDA approved the custom 3D-printed splints for use in Justice. She underwent implantation of the devices on October 6, 2020, in a multidisciplinary surgery involving cardiology, otolaryngology, and pulmonology. One month later, after gradual weaning from oxygen, Justice went home with her parents and sister.
Advancing the Treatment of Tracheomalacia
Within 18 months of surgery, Justice, a child who’d previously been dependent on a feeding tube for nutrition, began enjoying food. It took time—and multiple speech therapy sessions—because Justice, like many children who have been intubated since birth, had developed an oral aversion and didn’t want anything near her mouth at first. Surgical placement of the custom 3D-printed external tracheal splints allowed her to avoid a tracheostomy and enjoy food and freedom of movement.
That’s a significant step forward in the treatment of severe tracheomalacia. Initially, custom-printed 3D-airway splints were only used to treat patients who were extremely unstable despite tracheostomies and mechanical ventilation. But as physicians witnessed the positive impact external airway splints could have on children and
families affected by severe tracheomalacia, they started offering surgical implantation of the splints as a primary intervention to Justice and other patients.
Hurdles remain. “One thing we’re working through now is the speed at which we can make this happen,” Dr. Goudy said. “Right now, it takes six to eight weeks, at a minimum, to go through all the hoops, design, 3D print, sterilize, and then implant custom splints.”
Currently, the FDA must still individually consider each case. Full FDA approval of the custom-printed splints is the eventual goal, Dr. Maher said, noting that he, Dr. Goudy, and Dr. Hollister have already met with the FDA to discuss what’s needed to hit that goal and ultimately increase access to customized external airway splints.
Justice is thriving today because physicians and engineers collaborated to create an innovative solution. That kind of creativity and collaboration can help advance the treatment of all kinds of medical conditions.
“As otolaryngologists, we need to continue to tell the story of the unmet needs of our patients,” Dr. Goudy said. “We need to communicate that to engineers and scientists and work alongside them to develop new therapies.”
Jennifer Fink is a freelance medical writer based in Wisconsin.