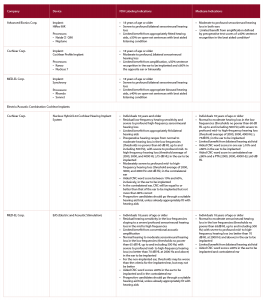
CMS, Centers for Medicare & Medicaid Services;
CNC, consonant-vowel nucleus-consonant;
FDA, Food and Drug Administration
a Medicare is considering expanding criteria to include those individuals with more residual hearing—≤40% sentence recognition in the ear to be implanted and ≤60% in the opposite ear.
Table 1: Comparison of Cochlear Implant Candidacy between FDA-Approved Devices and Medicare Adult Criteria
ENTtoday - https://www.enttoday.org/article/cochlear-implants-changing-indications-new-technology/ent_1117_pg8c/