Presentation: A 53-year-old man presented with a one-month history of bilateral tinnitus. Magnetic resonance imaging (MRI) performed at another hospital detected a sphenoid tumor incidentally. The patient had no nasal symptoms, and endoscopic observation did not confirm a tumor in the nasal cavity. A contrast-enhanced computed tomography (CT) scan revealed a partially enhanced tumor in the left sphenoidal sinus that had destroyed part of the skull base (Figure 1).
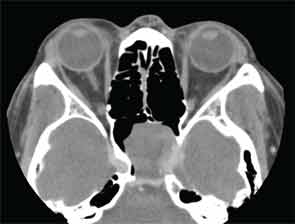
Figure 1: CT scan showing partially enhanced tumor in left sphenoidal sinus.
The tumor also involved the internal carotid artery and contacted the optic nerve canal. T1/T2-weighted MRI showed a hypointense tumor, and a gadolinium-enhanced T1-weighted image showed a rounded mass. Although the patient experienced no visual abnormalities, a malignant tumor was suspected from the imaging findings.
—Submitted by Shuji Yokoyama, MD, Hiroshi Ogawa, MD, Takashi Matsuzuka, MD, Mika Nomoto, MD, and Koichi Omori, MD, department of otolaryngology, Fukushima Medical University School of Medicine, Fukushima, Japan.
What’s your diagnosis? How would you manage this patient? Go to the next page for discussion of this case.
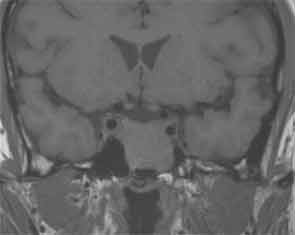
Figure 2: T1-weighted image showed a rounded mass.
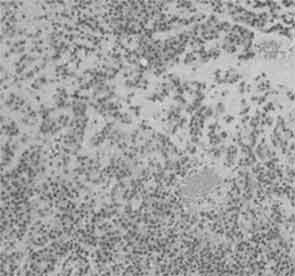
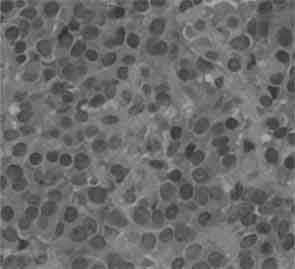
Figures 3 and 4: Histological examination showed plasmacytoid cells with eccentric nuclei and dense eosinophillic cytoplasm, and epithelioid cells with centrally located nuclei.
Management
An endoscopic biopsy was performed under general anesthesia. The tumor was cystic with a flat surface. When the tumor surface was incised, a pulsatile flow of blackish-brown liquid came from the mass. The tumor was incompletely excised because it involved the internal carotid artery and contacted the optic nerve canal. There were no post-operative complications, and at 96 months, we have found no evidence of tumor growth.
Pathological Findings
Histological examination showed plasmacytoid cells with eccentric nuclei and dense eosinophilic cytoplasm and epithelioid cells with centrally located nuclei. Adherent tumor cells were arranged in sheets and were growing into the connective tissue fascicles (Figures 3 and 4). Chromatin staining of nuclei was weak, mitotic activity was extremely low, psammoma bodies were noted and bone destruction was slight. There was no invasion of tumor cells into the surrounding tissue. No ductal component, necrosis or lymphovascular invasion was present.
On immunohistochemical staining, tumor cells were positive for cytokeratin (AE1/AE3), chromogranin, neuron-specific enolase (NSE) and synaptophysin, but negative for S-100 protein, vimentin and glial fibrillary acidic protein (GFAP). Thus, the histological diagnosis was myoepithelioma.
Discussion
Myoepithelioma is a tumor derived from myoepithelial cells in the interstitial segment of the exocrine gland. This neoplasm generally arises in the mammary gland and trachea and manifests as a salivary gland tumor.1 The tumor most commonly occurs in the parotid salivary gland, yet it accounts for less than 1 percent of all salivary gland neoplasms, and only extremely rarely does it occur in the nasal cavity.2 Although myoepithelioma and pleomorphic adenoma behave similarly, myoepithelioma is histologically distinguished from pleomorphic adenoma by the presence of a myxoma-like structure and lack of ductal differentiation.
Myoepithelioma was first reported by Sheldon in 1943, and in 1991 the World Health Organization classification first recognized this salivary gland tumor as a specific entity, with the revised edition published in 2005.3,4 Myoepithelial cells lie between the epithelium and basal cells in intercalated ducts and acini of exocrine glands.5 Nasal and sinus myoepithelioma is an extremely rare neoplasm that represents less than 1 percent of all salivary gland neoplasms. Most myoepitheliomas arise from an embryonic nest of minor salivary gland tissue. There is no sex predilection, and myoepithelioma is most commonly seen in adults.4
Findings on both electron and light microscopy and immunohistochemical examination are important for the diagnosis of myoepithelioma. An electron microscopy study revealed the characteristic presence of myofilaments and tonofilaments, and myoepithelioma cells are classified into four types histologically: spindle, plasmacytoid, epithelioid and clear cell. Most tumor cells consist of one or a combination of these four cell types.5,6 Immunohistochemically, myoepithelial cells can be characterized by a positive reaction for S-100 protein, vimentin and cytokeratin, and a weak reaction for GFAP.7-9
Additionally, S-100 protein and vimentin are positive in neoplastic myoepithelial cells and negative in normal myoepithelial cells. However, neoplastic transformation of myoepithelial cells causes variable positivity.4 Furthermore, salivary gland myoepitheliomas differ from soft-tissue myoepitheliomas in the expression of some markers.9 Hagisawa and colleagues found that S-100 protein, vimentin and chromogranin A were negative in middle-ear myoepithelioma.10 Current reports suggest that surgical extirpation of myoepithelioma is associated with a good prognosis and that radiotherapy and chemotherapy were ineffective treatments.
We found only nine detailed cases of myoepithelioma of the sinonasal tract reported worldwide.11-18 Myoepithelioma of the nasal cavity tends to occur in females with the origin in or on the nasal septum (3), turbinate (3), maxillary sinus (1), ethmoid sinus (1) and piriform aperture (1). Surgery was performed in all nine previously reported cases, and radiotherapy was used in three cases.
Immunohistochemically, tumor cells in our case were positive for cytokeratin (AE1/AE3), chromogranin, NSE and synaptophysin but negative for S-100 protein, vimentin and GFAP. Based on these findings, we first suspected a neuroendocrine tumor such as a paraganglioma or olfactory neuroblastoma. However, because the tumor was negative on hematoxylin and eosin staining, our diagnosis was a variant of myoepithelioma. Also, we saw no cellular atypia, and the tumor did not invade the surrounding tissue, but there was some bone destruction. Therefore, the tumor was classified as a low-grade malignancy. In our case, because the tumor involved the internal carotid artery and contacted the optic nerve canal, tumor extirpation and radiotherapy were avoided to prevent the risk of optic nerve disorder, and the patient was followed closely.
Differential diagnosis of myoepithelioma includes pleomorphic adenoma and other salivary gland tumors. Myoepithelioma is histologically distinct from pleomorphic adenoma due to the presence of a myxoma-like structure and absence of ductal differentiation.6,19 Although myoepithelioma has fewer recurrences than pleomorphic adenoma, benign myoepitheliomas can transform into malignancies.20 No evidence of tumor growth at 96 months was seen.
Conclusion
Myoepithelioma originating from the sphenoidal sinus is extremely rare, and both hematoxylin and eosin staining, along with immunohistochemical examination, were crucial for diagnosis. In our case, the tumor was classified as a low-grade malignancy, but we did not extirpate the tumor in order to avoid surgical complications. Under watchful waiting, there has been no evidence of tumor growth at 96 months.
References
- Venkatraman L, Sinnathuray AR, Raut V, Brooker DS, McCluggage WG. Soft tissue myoepithelioma: a case report. Pathology. 2002;34:451-454.
- Sciubba JJ, Brannon RB. Myoepithelioma of salivary glands: report of 23 cases. Cancer. 1982;49:562-572.
- Sheldon WH. So-called mixed tumors of the salivary glands. Arch Pathol. 1943;35:1-20.
- Cardesa A, Alos L. Myoepithelioma. In: Barnes L, Everson JW, Reichert P, Sidransky D, eds. Pathology and Genetics of Head and Neck Tumours. Tumours of the Salivary Glands. World Health Organization. Lyon: IARC Press;2005:259-260.
- Michal M, Miettinen M. Myoepitheliomas of the skin and soft tissues. Report of 12 cases. Virchows Arch. 1999;434:393-400.
- Dardick I, Thomas MJ, van Nostrand AW. Myoepithelioma-new concepts of histology and classification: a light and electron microscopic study. Ultrastruct Pathol. 1989;13:187-224.
- Lateef SS, Castillo M, Mukherji SK, Cooper LL. Myoepithelioma of the nasal piriform aperture: CT findings. AJR Am J Roentgenol. 1999;173:1413-1414.
- Dardick I, Cavell S, Boivin M, et al. Salivary gland myoepithelioma variants. Histological, ultrastructural, and immunocytological features. Virchows Arch A Pathol Anat Histopathol. 1989;416:25-42.
- Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am J Surg Pathol. 2003;27:1183-1196.
- Hagisawa M, Yamasoba T, Ohashi K, Ishida T. A case of middle-ear myoepithelioma. Otolaryngol Head Neck Surg. 2006;135:967-968.
- Mori M, Ninomiya T, Okada Y, Tsukitani K. Myoepitheliomas and myoepithelial adenomas of salivary gland origin. Immunohistochemical evaluation of filament proteins, S-100 alpha and beta, glial fibrillary acidic proteins, neuron-specific enolase, and lactoferrin. Pathol Res Pract. 1989;184:168-178.
- Bégin LR, Black MJ. Salivary-type myxoid myoepithelioma of the sinonasal tract: a potential diagnostic pitfall. Histopathology.1993;23:283-285.
- Bégin LR, Rochon L, Frenkiel S. Spindle cell myoepithelioma of the nasal cavity. Am J Surg Pathol. 1991;15:184-190.
- Hsiao CH, Cheng CJ, Yeh KL. Immunohistochemical and ultrastructural study of malignant plasmacytoid myoepithelioma of the maxillary sinus. J Formos Med Assoc. 1997;96:209-212.
- Nakaya K, Oshima T, Watanabe M, et al. A case of myoepithelioma of the nasal cavity. Auris Nasus Larynx. 2010;37:640-643.
- Kobayashi Y, Katayama A. Myoepithelioma of the nasal cavity; a case report. Pract Otologica (Kyoto). 1998;91:691-694.
- Tateya I, Sugimaru T, Fujiki N, Kurata K. Myoepithelioma of the paranasal sinus; a case report. Pract Otologica (Kyoto). 1996;89:1359-1364.
- Uno Y, Maeta M, Saito R, Kanatani M. Malignant Myoepithelioma of the nasal cavity; a case report. Pract Otologica (Kyoto). 1994;87:351-355.
- Bolzoni A, Pianta L, Farina D, Nicolai P. Benign myoepithelioma of the lacrimal gland: report of a case. Eur Arch Otorhinolaryngol. 2005;262:186-188.
- Seifert G, Sobin LH. The World Health Organization’s Histological Classification of Salivary Gland Tumors. A commentary on the second edition. Cancer. 1992;70:379-385.
Leave a Reply