Explore This Issue
May 2011For decades, otolaryngologists have been frustrated by the refusal of some patients with hearing loss to use hearing aids. Statistics on noncompliance vary, but there is general agreement that only about 20 percent to 25 percent of Americans with treatable hearing loss use hearing aids (Hear Rev. 2009;16(11):12-31). The problem seems to be more acute for people with mild hearing loss: A consumer survey conducted by the nonprofit Better Hearing Institute in 2009 found that fewer than 10 percent of people with mild hearing loss use amplification and that even among people with moderate-to-severe hearing loss, only four in 10 use amplification (Hear Rev. 2010;17(6):12,14-16).
Non-users offer many reasons for going unaided, including the high cost of hearing aids, the inconvenience of wearing them, the fear of seeming old, and negative reports from friends and family who have tried them. But an emerging category of hearing devices, middle ear implants (MEIs), may offer a solution to the noncompliance problem, experts say.
“If you have a patient whose hearing is not bad enough for a cochlear implant but who is really dissatisfied with hearing aids, that is a niche for implantable devices,” said Moisés A. Arriaga, MD, MBA, FACS, who has participated in clinical trials of several models of MEIs. Dr. Arriaga is professor of otolaryngology and neurosurgery at Louisiana State University and director of Our Lady of the Lake Hearing and Balance Center in Baton Rouge, La.
Options
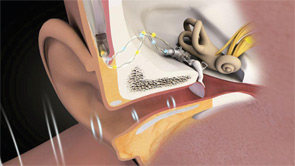
Patients can choose between partially implantable and totally implantable systems. In the former, the receiver is implanted on the ossicular chain, while the microphone, battery and, in some cases, the processor are external. In the latter, every component is surgically implanted except for a remote control that lets the wearer adjust the volume and turn the device on and off.
Currently, three companies have U.S. Food and Drug Administration (FDA)-approved middle ear implants: MED-EL, an Austria-based company that in 2003 purchased the Vibrant Soundbridge technology from Symphonix; Ototronix, which makes the Maxum System, a device based on SOUNDTEC Inc.’s FDA-approved Direct System; and Envoy Medical Corp., of Minneapolis, which received FDA clearance for its totally implantable Esteem system in March 2010. A fourth company, Otologics, has both semi-implantable and totally implantable systems on the market in Europe, but neither has been approved in the U.S.
Leave a Reply