Reprinted from the August 15 Neurology Today with permission from the American Academy of Neurology.
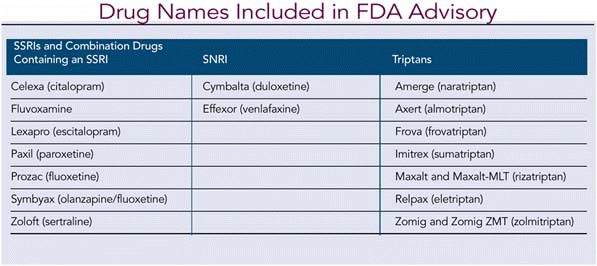
A new Food and Drug Administration (FDA) advisory warns that the combined use of triptans and selective serotonin reuptake inhibitors (SSRIs) or selective serotonin/norepinephrine reuptake inhibitors (SNRIs) may result in life-threatening serotonin syndrome, which occurs when the body has too much serotonin.
Migraine experts maintain that this risk is small, however, and they do not advocate stopping the combined use of these drugs for people with migraine and the comorbidity of depression and anxiety in the absence of serotonin syndrome symptoms. They do suggest, though, that clinicians monitor and discuss the risk with their patients. (See “Symptoms of Serotonin Syndrome.”)
“I understand the FDA’s obligation to the public safety,” said Stewart J. Tepper, MD, Associate Clinical Professor of Neurology at Yale University School of Medicine and Director of the New England Center for Headache. The risk of a life-threatening event when using these drugs may not be zero; however, migraine and the comorbidity of anxiety and depression are prevalent and people need treatment, he said. “I don’t want to say the serotonin syndrome isn’t serious, but its occurrence in combination is highly unlikely,” he added.
Asked to comment, Crystal Rice, an FDA spokesperson, acknowledged that the risk is small, but she said that “knowing about it may help patients and physicians recognize this event, if it does occur, and may facilitate appropriate management of the patient.”
Data Summary and Advisory
The FDA reviewed 27 cases of serotonin syndrome reported from 1998 to 2002 in association with concomitant SSRI or SNRI and use of triptans. Two reports involved life-threatening events, none fatal, and 13 patients required hospitalization. Some cases occurred in patients who had previously used concomitant SSRIs or SNRIs and triptans without experiencing serotonin syndrome.
The signs and symptoms of serotonin syndrome were highly variable and included respiratory failure, coma, mania, hallucinations, confusion, dizziness, hyperthermia, hypertension, sweating, trembling, weakness, and ataxia.
In eight cases, recent dose increases or the addition of another serotonergic drug to an SSRI/triptan or SNRI/triptan combination were temporally related to symptom onset. The median time to onset subsequent to the addition of another serotonergic drug or dose increase was one day, with a range of 10 minutes to 6 days.
“SSRIs, SNRIs, and triptans independently increase serotonin levels,” wrote the FDA. “Therefore, it is expected that concomitant use of SSRIs or SNRIs and triptans would result in higher serotonin levels than the serotonin levels observed with the use of SSRIs, SNRIs, or triptans alone, potentially leading to serotonin syndrome.”

Leave a Reply