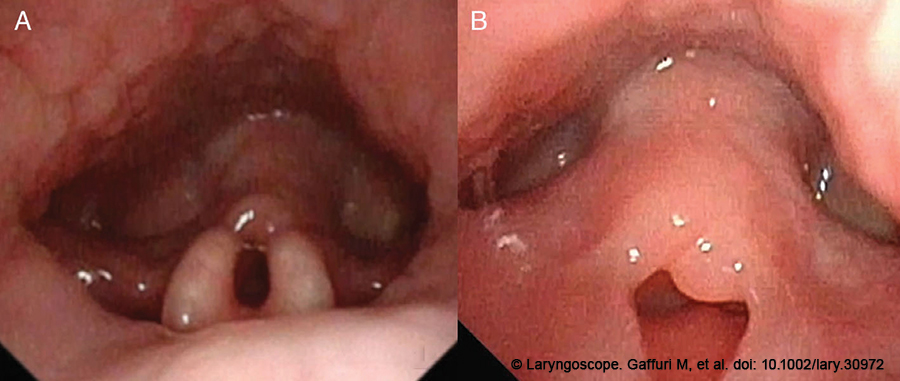
Figure 1. The diagnosis of laryngomalacia type II was made by means of an awake flexible HD videolaryngoscopy: a curled tubular epiglottis (A), short aryepiglottic folds and redundant supra-arytenoid floppy mucosa (B), were clearly visible during the examination.
INTRODUCTION
Laryngomalacia (LM) is the most common congenital laryngeal anomaly caused by a delay in the maturation of supporting laryngeal cartilages, leading to inward collapse of the supraglottic structures during inspiration (Eur Arch Otorhinolaryngol. 2017. doi: 10.1007/s00405-016-4252-6). The main symptom is a high-pitched, fluttering inspiratory stridor, which intensifies with increased airway demands (crying, feeding) or when the patient is in a supine position. Typically, the disease is self-limiting, with onset between two and four weeks of age and spontaneous resolution occurring by two years.
Explore This Issue
December 2023Adjunctive symptoms such as regurgitation, vomiting, coughing, or choking may occur in severe cases (Eur Arch Otorhinolaryngol. 2017. doi: 10.1007/s00405-016-4252-6). Diagnosis is clinical and endoscopic by means of awake flexible videolaryngoscopy; severe LM cases presenting with stridor, compromised airway, feeding difficulties, failure to thrive, and obstructive sleep apnea are amenable to a surgical intervention with supraglottoplasty (Eur Arch Otorhinolaryngol. 2017. doi: 10.1007/s00405-016-4252-6).
Traditionally, surgery is performed using a microscope and cold knife, carbon dioxide (CO2) laser, microdebrider or coblation (Eur Arch Otorhinolaryngol. 2017. doi: 10.1007/s00405-016-4252-6). Recently, the use of three-dimensional (3D) exoscopy has been reported in head and neck surgery, including laryngeal surgery for adult patients, with satisfactory and promising results; however, only a few pediatric series have been published so far, focusing primarily on ear surgery (J Clin Med. 2022. doi: 10.3390/jcm11133639; Eur Arch Otorhinolaryngol. 2023. doi: 10.1007/s00405-022-07785-x).
The main advantages of 3D-exoscopy over microscopy and conventional endoscopy, are depth of the surgical field, the 3D visualization of anatomical structures, and a longer focal length creating a wider working space, improved camera handling thanks to the combined use of a mechanical arm, the ARTip Cruise, allowing the surgeon to explore the surgical field without the anatomical limitations of microscopy (J Clin Med. 2022. doi: 10.3390/jcm11133639; Eur Arch Otorhinolaryngol. 2023. doi: 10.1007/s00405-022-07785-x). Moreover, surgery can be performed in a more ergonomic position thanks to the transmission of the images on a 4K 3D screen, placed at the eye level of the main surgeon, reducing the physical and mental demand and frustration for the surgeon; this setting facilitates the transmission of knowledge to students, and can be used for training, thanks also to the possibility of creating videos for the surgical training (Eur Arch Otorhinolaryngol. 2023. doi: 10.1007/s00405-022-07785-x).
To the best of our knowledge, the use of a 3D-4K exoscope for laryngeal surgery on pediatric patients has not been previously reported in the literature. We here describe the use of 3D-4K exoscope-assisted CO2 laser supraglottoplasty under tubeless general anesthesia in spontaneous breathing in a five-month-old patient with severe laryngomalacia.
METHOD
The patient was a five-month-old female born from an uneventful full-term vaginal delivery with inspiratory stridor, feeding difficulties, and failure to thrive since birth.
The diagnosis of laryngomalacia type II, according to Olney et al. (Laryngoscope. 1999. doi: 10.1097/00005537-199911000-00009), with curled tubular epiglottis, short aryepiglottic (AE) folds, and redundant supra-arytenoid floppy mucosa, collapsing into the glottic inlet during inspiration, was made via an awake flexible HD videolaryngoscopy (Fig. 1A,B); on the basis of the patient’s symptoms and the diagnosis of LM, a 3D–4K exoscope-assisted laser supraglottoplasty was planned.
After multidisciplinary discussion with the pediatric anesthesiology team to determine the optimal airway management strategy, the patient was admitted and scheduled for a diagnostic and operative direct laryngotracheoscopy (DL) under general tubeless anesthesia in spontaneous breathing. At the time of surgery, the patient weighed 7 kg. The anesthetic technique used was standardized as previously described (Acta Otorhinolaryngol Ital. 2020. doi: 10.14639/0392-100X-N0830). Glottic anesthesia was performed by means of lidocaine sprayed into the larynx via an atomizer under direct laryngoscopic vision at a dose of 2–4 mg/kg. Under endoscopic guidance, supplemental oxygen was administered through a Portex Blue Line catheter inserted into the hypopharynx via a nostril.
The patient was suspended on a pediatric Lindholm laryngoscope inserted into the valleculae, allowing an axial, panoramic and symmetrical exposure of the supraglottic structures. A DL was performed using a 0- to 4-mm Storz-Hopkins rod endoscope; the diagnosis of laryngomalacia type II according to Olney et al. (Laryngoscope. 1999. doi: 10.1097/00005537-199911000-00009), was confirmed; a tracheoscopy performed with a 0- to 2.7-mm Storz-Hopkins rod endoscope showed a normal tracheal and main bronchi lumen with no adjunctive anomalies detectable. The Vitom 3D exoscope was used in combination with the ARTip Cruise mechanical support system and positioned at the head of the patient, with the respective monitor set at the patient’s feet. The 3D–4K exoscope was aligned with the laryngoscope.
The procedure was performed using a CO2 laser connected to the exoscope and guided by a micromanipulator. The laser parameters used were: 10 W ultrapulse, 0.20 s repetition frequency, 1 mm depth, linear laser spot, length 1 mm. Surgery was performed by visualizing the surgical field in a 3D fashion by means of 3D glasses on a 4K 32-inch screen. Supraglottoplasty type II was performed: the short AE folds were sectioned, floppy supraglottic tissues were partially resected and/or vaporized and the lateral edges of the epiglottis were reshaped. At the end of surgery, a DL was performed using a 0- to 4-mm Storz-Hopkins rod endoscope to evaluate the outcome.
RESULTS
The entire procedure lasted approximately 60 minutes, and no major or minor complications occurred during or immediately after surgery. Temporary adrenaline and budesonide aerosols were administered in the immediate postoperative period. Dexamethasone (1 mg/kg), cefazoline (25 mg/kg) and proton pump inhibitors were administered in the operating room and were continued during the postoperative period.
The patient was then admitted to the Pediatric Intensive Care Unit for 24 hours; no oxygen desaturations were noticed, and breastfeeding was resumed three hours after surgery. Three days later, the patient was discharged without any respiratory symptoms.
Two weeks after surgery, clinical and endoscopic follow-up was performed: the patient did not present any respiratory symptoms and an HD videolaryngoscopy clearly demonstrated complete healing of laryngeal structures, with no evidence of redundant mucosa collapsing into the glottic space.